Silylene on:
[Wikipedia]
[Google]
[Amazon]
Silylene is a
 The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
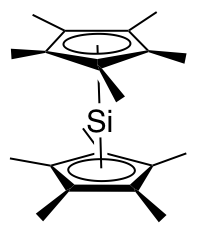 In one study diphenylsilylene is generated by flash photolysis of a trisilane:
:
In one study diphenylsilylene is generated by flash photolysis of a trisilane:
: In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula SiH2. It is the silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
analog of methylene, the simplest carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.
Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents. Most examples feature amido (NR2) or alkyl/aryl groups.
Silylenes have been proposed as reactive intermediates. They are carbene analogs.
Synthesis and properties
Silylenes are generally synthesized by thermolysis or photolysis ofpolysilane
Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly use ...
s, by silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
atom reactions ( insertion, addition or abstraction), by pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
of silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Sila ...
s, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates:
:Si + Cl2 → SiCl2
:SiCl2 + Cl2 → SiCl4
Similar considerations apply to the direct process, the reaction of methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industria ...
and bulk silicon.
Early observations of silylenes involved generation of dimethylsilylene by dechlorination of dimethyldichlorosilane:
:SiCl2(CH3)2 + 2 K → Si(CH3)2 + 2 KCl
The formation of dimethylsilylene was demonstrated by conducting the dechlorination in the presence of trimethylsilane
Trimethylsilane is the organosilicon compound with the formula (CH3)3SiH. It is a trialkylsilane. The Si-H bond is reactive. It is less commonly used as a reagent than the related triethylsilane, which is a liquid at room temperature.
Trimethyls ...
, the trapped product being pentamethyldisilane:
:Si(CH3)2 + HSi(CH3)3 → (CH3)2Si(H)−Si(CH3)3
A room-temperature isolable ''N''-heterocyclic silylene is , first described in 1994 by Michael K. Denk et al.
 The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.
Related reactions
 In one study diphenylsilylene is generated by flash photolysis of a trisilane:
:
In one study diphenylsilylene is generated by flash photolysis of a trisilane:
: In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with
In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with UV spectroscopy
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
at 520 nm and is short-lived with a chemical half-life of two microsecond
A microsecond is a unit of time in the International System of Units (SI) equal to one millionth (0.000001 or 10−6 or ) of a second. Its symbol is μs, sometimes simplified to us when Unicode is not available.
A microsecond is equal to 1000 ...
s. Added methanol acts as a chemical trap with a second order rate constant of which is close to diffusion control.
See also
* Carbene analogs * ''N''-heterocyclic silylene *Silenes
Silene, or disilalkenes,Philip P. Power "pi-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements" Chemical Reviews, 1999, 99, 3462. are silicon compounds that contain Si=Si double bonds. The parent silene is ( ...
, R2Si=SiR2
*Silylium ion
A silylium ion is a reactive silyl-containing cation with the formula . With three rather than the usual four bonds to Si, silylium ions are the silicon analogues of carbenium ions. They can be viewed as protonated silylenes. Early efforts to gene ...
s, protonated silylenes
References
{{Reflist Inorganic silicon compounds Free radicals