|
Michael K. Denk
Michael K. Denk (or Karl Michael Denk) is a Professor of chemistry at the University of Guelph, Ontario.Michael K. Denk'Personal Home Page at myprofile.cos.com. Accessed on 2009-12-07. Karl Michael Denk (1992): ''Cyclische Metallamide : Synthese, Strukturchemie, Stabilität''. Ph. D. Thesis, TU MünchenNEBIS library catalog entry Accessed on 2009-12-07. Michael Denk obtained his M.Sc. at the Ludwig Maximilian University in Munich, Germany (1989) and his Ph.D. at the Technical University of Munich (1992), advised by W. Herrmann, W. A. Herrmann'list of advised Ph.D. studentsat TU-Munich. Accessed on 2009-12-07. with a dissertation on cyclic metalloamides. He has previously held academic positions at the University of Toronto (1995-2001), Purdue University (1994–1995), and the University of Wisconsin-Madison (1993–1994) and was head of research on Tibetan ethnobotany at Klinge Holding & Research. Michael Denk's research ranges over several areas of organic and inorganic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klinge Holding & Research
Klinge (''Lower Sorbian: Klinka'') is a village in the Lower Lusatia region, east of the city of Cottbus in Brandenburg. It is part of the municipality of Wiesengrund. Transport The Bahnhof Klinge is served once an hour in each direction by the RB46 train travelling between Cottbus and Forst Forst may refer to: Communities In Germany *, in the district of Aachen *Forst (Baden), in Baden-Württemberg *Forst (Lausitz), in Brandenburg * Forst (Unterfranken), part of Schonungen, Bavaria * Forst, Altenkirchen, in the district of Altenk .... References External links A train at Bahnhof Klinge Villages in Brandenburg Populated places in Spree-Neiße {{Brandenburg-geo-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wanzlick Equilibrium
The Wanzlick equilibrium is a chemical equilibrium between a relatively stable carbene compound and its dimer. The equilibrium was proposed to apply to certain electron-rich alkenes, such as tetraminoethylenes, which have been called "carbene dimers." Such equilibria occur, but the mechanism does not proceed simply, but requires catalysts. Original conjecture In 1960, Hans-Werner Wanzlick and E. Schikora proposed that carbenes derived from dihydroimidazol-2-ylidene were generated by vacuum pyrolysis of 2-trichloromethyl dihydroimidazole derivatives, with the loss of chloroform. Wanzlick and Schikora believed that once prepared these carbenes existed in an unfavourable equilibrium with their corresponding dimers. This assertion was based on reactivity studies which they believed showed that the free carbene reacted with electrophiles (E-X). The dimer (a substituted tetraaminoethylene) was believed to be inactive to the electrophiles (E-X), and thought to merely act as a stable c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thin Film
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, LEDs, optical coatings (such as antireflective coatings), hard coatings on cutting tools, and for both energy generation (e.g. thin-film solar cells) and storage ( thin-film batteries). It is also being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glass. Its electrical resistivity and conductivity, resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping (semiconductor), doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (substrate) is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: monocrystalline, polycrystalline, amorphous, and epitaxial. These materials include: silicon ( dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond and graphene), fluorocarbons, filaments, tungsten, titanium nitride and various high-κ dielectrics. The term ''chemical vapour deposition'' was coined 1960 by ''John M. Blocher, Jr.'' who intended to differentiate ''chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
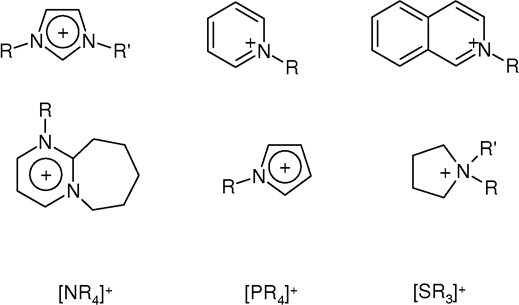
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–750 nm) or infrared radiation (750–2500 nm). In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation of plastics. Concept Grotthuss–Draper law and Stark-Einstein law Photoexcitation is the first step in a photochemical process where the reactant is elevated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganics, metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature. Because it seldom appears in high concentration, germanium was discovered comparatively late in the discovery of the elements. Germanium ranks near fiftieth in relative abundance of the elements in the Earth's crust. In 1869, Dmitri Mendeleev predicted its existence and some of its properties from its position on his periodic table, and called the element ekasilicon. In 1886, Clemens Winkler at Freiberg University found the new element, along with silver and sulfur, in the mineral argyrodite. Winkler named the element after his country, Germany. Germanium is mined primarily from sphalerite (the primary ore of zinc), though germanium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron. Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_250_nm_by_250_nm_image_of_one-atom-thick_silver_islands_grown_on_palladium_(111)_surface.png)