|
1,3-Dioxane
1,3-Dioxane or ''m''-dioxane is a chemical compound with the molecular formula C4H8O2. It is a saturated six-membered heterocycle with two oxygen atoms in place of carbon atoms at the 1- and 3- positions. The corresponding five-membered rings are known as 1,3-dioxolanes. Like 1,3-dioxolanes, 1,3-dioxanes are acetals which can be used as protecting groups for carbonyl compounds. They are prepared from the reaction between carbonyl compounds (formaldehyde for the parent 1,3-dioxane) and 1,3-propanediol in the presence of Brönsted or Lewis acid catalysts. See also * 1,2-Dioxane * 1,4-Dioxane *Dithiane A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane. 1,3- ... References Dioxanes Cyclic ethers {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4-Dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- and 1,3-) are rarely encountered. Dioxane is used as a solvent for a variety of practical applications as well as in the laboratory, and also as a stabilizer for the transport of chlorinated hydrocarbons in aluminum containers.Wisconsin Department of Health Services (20131,4-Dioxane Fact Sheet Publication 00514. Accessed 2016-11-12. Synthesis Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn is obtained from the hydrolysis of ethylene oxide. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 5,250 and 9,150 tons. Structure The dioxane molecule is centrosymmetric, meaning that it adopts a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxanes
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- and 1,3-) are rarely encountered. Dioxane is used as a solvent for a variety of practical applications as well as in the laboratory, and also as a stabilizer for the transport of chlorinated hydrocarbons in aluminum containers.Wisconsin Department of Health Services (20131,4-Dioxane Fact Sheet Publication 00514. Accessed 2016-11-12. Synthesis Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn is obtained from the hydrolysis of ethylene oxide. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons. In 1990, the total U.S. production volume of dioxane was between 5,250 and 9,150 tons. Structure The dioxane molecule is centrosymmetric, meaning that it adopts a cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dioxane
1,2-Dioxane or ''o''-dioxane is an organic compound with the molecular formula (CH)O, classified as a cyclic peroxide. Its synthesis was reported in 1956 by Criegee and Müller, who prepared it by reacting butane-1,4-diol bis(methanesulfonate) with hydrogen peroxide and distilled it as a colorless liquid. Acids and bases decompose it to ''gamma''-hydroxybutyraldehyde. Substituted 1,2-dioxanes have also been prepared, and some have been isolated from natural sources. See also * 1,3-Dioxane * 1,4-Dioxane 1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2 ... References Dioxanes Organic peroxides {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
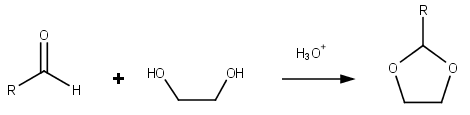
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection of carbonyls including as dioxolanes are kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. A s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-Interscience
John Wiley & Sons, Inc., commonly known as Wiley (), is an American multinational publishing company founded in 1807 that focuses on academic publishing and instructional materials. The company produces books, journals, and encyclopedias, in print and electronically, as well as online products and services, training materials, and educational materials for undergraduate, graduate, and continuing education students. History The company was established in 1807 when Charles Wiley opened a print shop in Manhattan. The company was the publisher of 19th century American literary figures like James Fenimore Cooper, Washington Irving, Herman Melville, and Edgar Allan Poe, as well as of legal, religious, and other non-fiction titles. The firm took its current name in 1865. Wiley later shifted its focus to scientific, technical, and engineering subject areas, abandoning its literary interests. Wiley's son John (born in Flatbush, New York, October 4, 1808; died in East Orange, New J ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiane
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane. 1,3-Dithianes 1,3-Dithianes are protecting group of some carbonyl-containing compounds due to their inertness to many conditions. They form by treatment of the carbonyl compound with 1,3-propanedithiol under conditions that remove water from the system. The protecting group can be removed with mercuric reagents, a process that exploits the high affinity of Hg(II) for thiolates. 1,3-Dithianes are also employed in umpolung reactions, such as the Corey–Seebach reaction:T. W. Green, P. G. M. Wuts, "Protective Groups in Organic Synthesis" Wiley-Interscience, New York, 1999. . : Typically, in organic synthesis Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |