Dioxolane on:
[Wikipedia]
[Google]
[Amazon]
Dioxolane is a
 (+)-''cis''-Dioxolane is the
(+)-''cis''-Dioxolane is the
 NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds. For example, deprotection of 2-phenyl-1,3-dioxolane to benzaldehyde can be achieved in water in five minutes at 30 °C.
::PhCH(OCH2)2 + H2O
NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds. For example, deprotection of 2-phenyl-1,3-dioxolane to benzaldehyde can be achieved in water in five minutes at 30 °C.
::PhCH(OCH2)2 + H2O -> ce\text] PhCHO + ethylene glycol, HOCH2CH2OH
 A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.
A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.
environmental and toxicological data
{{Muscarinic acetylcholine receptor modulators Muscarinic agonists Solvents Protecting groups Formals
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
(CH2)2O2CH2. It is related to tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
s. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
. 1,3-dioxolane is used as a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
and as a comonomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
in polyacetal
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. As with many other synthetic ...
s.
As a class of compounds
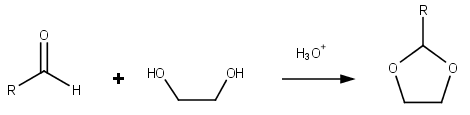
Dioxolanes are a group oforganic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s containing the dioxolane ring. Dioxolanes can be prepared by acetalization
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
of aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s and ketalization of ketones with ethylene glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
.
 (+)-''cis''-Dioxolane is the
(+)-''cis''-Dioxolane is the trivial name
In chemistry, a trivial name is a non systematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC inorganic or IUPAC organic nomenclature. A ...
for which is a muscarinic acetylcholine receptor
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-rec ...
agonist.
Protecting groups
Organic compounds containingcarbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
s sometimes need protection
Protection is any measure taken to guard a thing against damage caused by outside forces. Protection can be provided to physical objects, including organisms, to systems, and to intangible things like civil and political rights. Although th ...
so that they do not undergo reactions during transformations of other functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s that may be present. A variety of approaches to protection and deprotection of carbonyls including as dioxolanes are known. For example, consider the compound methyl cyclohexanone-4-carboxylate, where lithium aluminium hydride reduction will produce 4-hydroxymethylcyclohexanol. The ester functional group can be reduced without affecting the ketone by protecting the ketone as a ketal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
. The ketal is produced by acid catalysed reaction with ethylene glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
, the reduction reaction carried out, and the protecting group removed by hydrolysis to produce 4-hydroxymethylcyclohexanone.
 NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds. For example, deprotection of 2-phenyl-1,3-dioxolane to benzaldehyde can be achieved in water in five minutes at 30 °C.
::PhCH(OCH2)2 + H2O
NaBArF4 can also be used for deprotection of acetal or ketal-protected carbonyl compounds. For example, deprotection of 2-phenyl-1,3-dioxolane to benzaldehyde can be achieved in water in five minutes at 30 °C.
::PhCH(OCH2)2 + H2O Natural products
Neosporol is a natural product that includes a 1,3-dioxolane moiety, and is an isomer of sporol which has a 1,3-dioxane ring. Thetotal synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes i ...
of both compounds has been reported, and each includes a step in which a dioxolane system is formed using trifluoroperacetic acid
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula . It is a strong oxidizing agent for organic oxidation reactions, such ...
(TFPAA), prepared by the hydrogen peroxide – urea method. This method involves no water, so it gives a completely anhydrous peracid, necessary in this case as the presence of water would lead to unwanted side reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the yield of main product is reduced:
: + B ->[] P1
: + C ->[] P2
P1 is th ...
s.
:trifluoroacetic anhydride, + hydrogen peroxide - urea, → trifluoroperacetic acid, + +
In the case of neosporol, a Prilezhaev reaction
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909 ...
with trifluoroperacetic acid is used to convert a suitable allyl alcohol
Allyl alcohol ( IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula . Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material ...
precursor to an epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
, which then undergoes a ring-expansion reaction with a proximate carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
functional group to form the dioxolane ring.
 A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.
A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.
See also
*Dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
References
External links
environmental and toxicological data
{{Muscarinic acetylcholine receptor modulators Muscarinic agonists Solvents Protecting groups Formals