|
Side Reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the yield of main product is reduced: : + B ->[] P1 : + C ->[] P2 P1 is the main product if k1> k2. The by-product P2 is generally undesirable and must be Separation process, separated from the actual main product (usually in a Industrial separation processes, costly process). In organic synthesis B and C from the above equations usually represent different compounds. However, they could also just be different positions in the same molecule. A side reaction is also referred to as competing reaction when different compounds (B, C) compete for another reactant (A). If the side reaction occurs about as often as the main reaction, it is spoken of parallel reactions (especially in the kinetics, see below). Also there may be more complicated relationships: Compound A could reversibly but quickly react to substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the Reversible reaction, reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The Concept learning, concept of chemical equilibrium was developed in 1803, after Claude Louis Berthollet, Berthollet found that some chemical reactions are Reversible reaction, reversible. For any reaction mixture to exist at equilibrium, the reaction rate, rates of the forward and backward (reverse) reactions must be equal. In the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elementary Reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction intermediates have been detected or need to be postulated to describe the reaction on a molecular scale. An apparently elementary reaction may be in fact a stepwise reaction, i.e. a complicated sequence of chemical reactions, with reaction intermediates of variable lifetimes. In a unimolecular elementary reaction, a molecule dissociates or isomerises to form the products(s) :\mbox \rightarrow \mbox At constant temperature, the rate of such a reaction is proportional to the concentration of the species :\frac=-k mbox In a bimolecular elementary reaction, two atoms, molecules, ions or radicals, and , react together to form the product(s) :\mbox \rightarrow \mbox The rate of such a reaction, at constant temperature, is proportional t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex Reaction
Complex commonly refers to: * Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe ** Complex system, a system composed of many components which may interact with each other * Complex (psychology), a core pattern of emotions etc. in the personal unconscious organized around a common theme such as power or status Complex may also refer to: Arts, entertainment and media * Complex (English band), formed in 1968, and their 1971 album ''Complex'' * Complex (band), a Japanese rock band * ''Complex'' (album), by Montaigne, 2019, and its title track * ''Complex'' (EP), by Rifle Sport, 1985 * "Complex" (song), by Gary Numan, 1979 * Complex Networks, publisher of magazine ''Complex'', now online Biology * Protein–ligand complex, a complex of a protein bound with a ligand * Exosome complex, a multi-protein intracellular complex * Protein complex, a group of two or more associated polypeptide chains * Spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical mechanics, analytical dynamics and chemical equilibria. Physical chemistry, in contrast to chemical physics, is predominantly (but not always) a supra-molecular science, as the majority of the principles on which it was founded relate to the bulk rather than the molecular or atomic structure alone (for example, chemical equilibrium and colloids). Some of the relationships that physical chemistry strives to resolve include the effects of: # Intermolecular forces that act upon the physical properties of materials ( plasticity, tensile strength, surface tension in liquids). # Reaction kinetics on the rate of a reaction. # The identity of ions and the electrical conductivity of materials. # Surface science and electrochemistry of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Branch
A branch, sometimes called a ramus in botany, is a woody structural member connected to the central trunk of a tree (or sometimes a shrub). Large branches are known as boughs and small branches are known as twigs. The term ''twig'' usually refers to a terminus, while ''bough'' refers only to branches coming directly from the trunk. Due to a broad range of species of trees, branches and twigs can be found in many different shapes and sizes. While branches can be nearly horizontal, vertical, or diagonal, the majority of trees have upwardly diagonal branches. A number of mathematical properties are associated with tree branchings; they are natural examples of fractal patterns in nature, and, as observed by Leonardo da Vinci, their cross-sectional areas closely follow the da Vinci branching rule. Terminology Because of the enormous quantity of branches in the world, there are numerous names in English alone for them. In general however, unspecific words for a branch (such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 35 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detection Reaction
{{Unreferenced, date=March 2018 In general, detection is the action of accessing information without specific cooperation from with the sender. In the history of radio communications, the term "detector" was first used for a device that detected the simple presence or absence of a radio signal, since all communications were in Morse code. The term is still in use today to describe a component that extracts a particular signal from all of the electromagnetic waves present. Detection is usually based on the frequency of the carrier signal, as in the familiar frequencies of radio broadcasting, but it may also involve filtering a faint signal from noise, as in radio astronomy, or reconstructing a hidden signal, as in steganography. In optoelectronics, "detection" means converting a received optical input to an electrical output. For example, the light signal received through an optical fiber is converted to an electrical signal in a detector such as a photodiode. In steganography ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
By-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced. A by-product can be useful and marketable or it can be considered waste: for example, bran, which is a byproduct of the milling of wheat into refined flour, is sometimes composted or burned for disposal, but in other cases, it can be used as a nutritious ingredient in human food or animal feed. Gasoline was once a byproduct of oil refining that later became a desirable commodity as motor fuel. The plastic used in plastic shopping bags also started as a by-product of oil refining. In economics In the context of production, a by-product is the "output from a joint production process that is minor in quantity and/or net realizable value (NRV) when compared with the main products". Because they are deemed to have no influence on reported financial results, by-products do not receive allocati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer. Thermometers are calibrated in various Conversion of units of temperature, temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
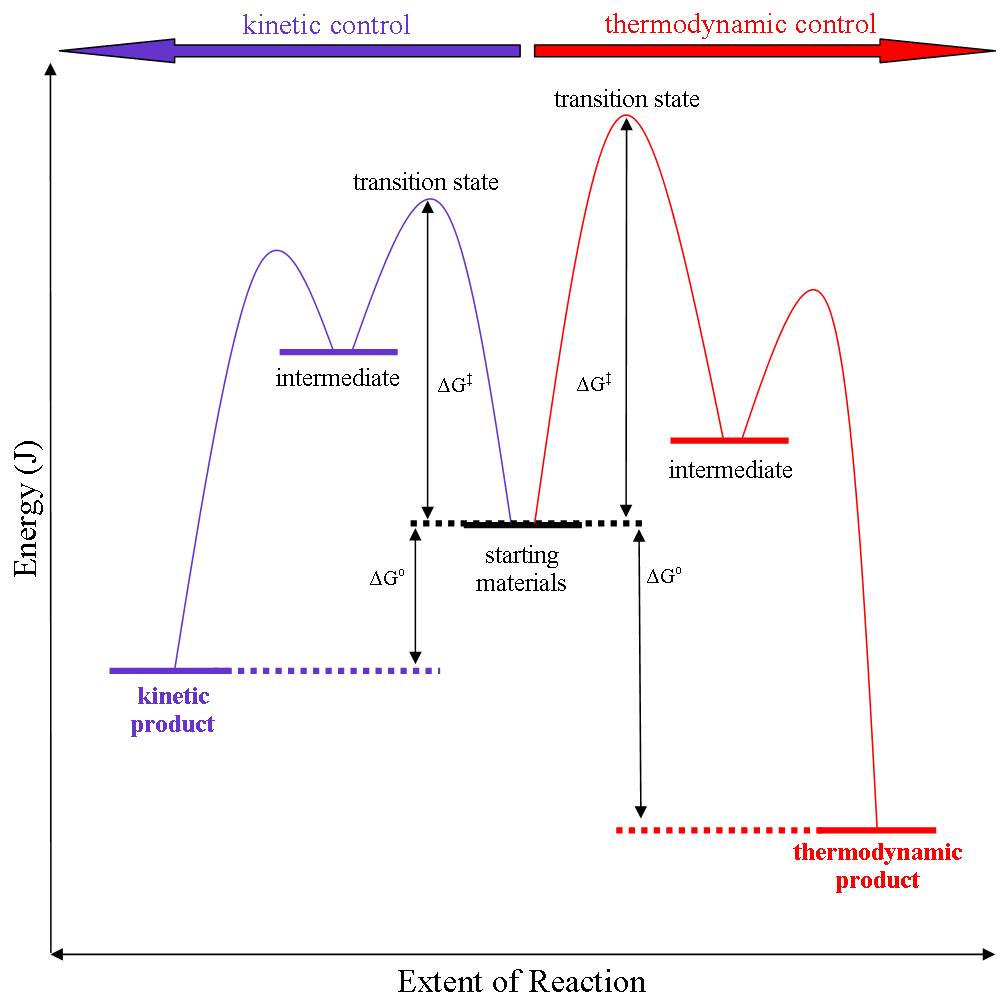
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)