Carbon dioxide (
chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
) is a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
made up of
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s that each have one
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atom
covalently double bonded to two
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs
infrared radiation, acting as a
greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
. It is a
trace gas in Earth's atmosphere at 421
parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm.
Burning
fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
s is the primary cause of these increased CO
2 concentrations and also the primary cause of
climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
.
[IPCC (2022]
Summary for policy makers
i
Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change
Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in
groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidate ...
,
lake
A lake is an area filled with water, localized in a basin, surrounded by land, and distinct from any river or other outlet that serves to feed or drain the lake. Lakes lie on land and are not part of the ocean, although, like the much large ...
s,
ice cap
In glaciology, an ice cap is a mass of ice that covers less than of land area (usually covering a highland area). Larger ice masses covering more than are termed ice sheets.
Description
Ice caps are not constrained by topographical features ...
s, and
seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
. When carbon dioxide dissolves in water, it forms
carbonic acid (H
2CO
3), which causes
ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
as
atmospheric CO2 levels increase.
As the source of available carbon in the
carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and Earth's atmosphere, atmosphere of the Earth. Carbon is the main component of biological compounds as well as ...
, atmospheric CO
2 is the primary carbon source for
life
Life is a quality that distinguishes matter that has biological processes, such as signaling and self-sustaining processes, from that which does not, and is defined by the capacity for growth, reaction to stimuli, metabolism, energ ...
on Earth. Its concentration in Earth's pre-industrial atmosphere since late in the
Precambrian
The Precambrian (or Pre-Cambrian, sometimes abbreviated pꞒ, or Cryptozoic) is the earliest part of Earth's history, set before the current Phanerozoic Eon. The Precambrian is so named because it preceded the Cambrian, the first period of the ...
has been regulated by organisms and geological phenomena.
Plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclud ...
s,
algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
and
cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blu ...
use
energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
from
sunlight
Sunlight is a portion of the electromagnetic radiation given off by the Sun, in particular infrared, visible, and ultraviolet light. On Earth, sunlight is scattered and filtered through Earth's atmosphere, and is obvious as daylight when t ...
to synthesize
carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s from carbon dioxide and water in a process called
photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, which produces oxygen as a waste product. In turn, oxygen is consumed and CO
2 is released as waste by all
aerobic organism
Aerobic means "requiring air," in which "air" usually means oxygen.
Aerobic may also refer to
* Aerobic exercise, prolonged exercise of moderate intensity
* Aerobics, a form of aerobic exercise
* Aerobic respiration, the aerobic process of cell ...
s when they metabolize
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s to produce energy by
respiration. CO
2 is released from organic materials when they
decay
Decay may refer to:
Science and technology
* Bit decay, in computing
* Software decay, in computing
* Distance decay, in geography
* Decay time (fall time), in electronics
Biology
* Decomposition of organic matter
* Tooth decay (dental caries) ...
or combust, such as in forest fires. Since plants require CO
2 for photosynthesis, and humans and animals depend on plants for food, CO
2 is necessary for the survival of life on earth.
Carbon dioxide is 53% more dense than dry air, but is long lived and thoroughly mixes in the atmosphere. About half of excess CO
2 emissions to the atmosphere are absorbed by
land and ocean
carbon sinks. These sinks can become saturated and are volatile, as decay and
wildfire
A wildfire, forest fire, bushfire, wildland fire or rural fire is an unplanned, uncontrolled and unpredictable fire in an area of Combustibility and flammability, combustible vegetation. Depending on the type of vegetation present, a wildfire ...
s result in the CO
2 being released back into the atmosphere. CO
2 is eventually
sequestered (stored for the long term) in rocks and organic deposits like
coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when dea ...
,
petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
and
natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
. Sequestered CO
2 is released into the atmosphere through burning fossil fuels or naturally by
volcano
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates are ...
es,
hot spring
A hot spring, hydrothermal spring, or geothermal spring is a spring produced by the emergence of geothermally heated groundwater onto the surface of the Earth. The groundwater is heated either by shallow bodies of magma (molten rock) or by circ ...
s,
geyser
A geyser (, ) is a spring characterized by an intermittent discharge of water ejected turbulently and accompanied by steam. As a fairly rare phenomenon, the formation of geysers is due to particular hydrogeological conditions that exist only in ...
s, and when
carbonate rocks
dissolve in water or react with acids.
CO
2 is a versatile industrial material, used, for example, as an inert gas in welding and
fire extinguishers, as a pressurizing gas in air guns and oil recovery, and as a supercritical fluid solvent in decaffeination of coffee and
supercritical drying.
It is also a
feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials that are feedstock for future finished products. As feedst ...
for the synthesis of fuels and chemicals. It is an unwanted byproduct in many large scale
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
processes, for example, in the production of
acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
(over 5 million tons/year). The frozen solid form of CO
2, known as
dry ice, is used as a refrigerant and as an abrasive in
dry-ice blasting
Dry-ice blasting is a form of carbon dioxide cleaning, where dry ice, the solid form of carbon dioxide, is accelerated in a pressurized air stream and directed at a surface in order to clean it.
The method is similar to other forms of media bla ...
. It is a byproduct of
fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
of sugars in
bread
Bread is a staple food prepared from a dough of flour (usually wheat) and water, usually by baking. Throughout recorded history and around the world, it has been an important part of many cultures' diet. It is one of the oldest human-made f ...
,
beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
and
wine
Wine is an alcoholic drink typically made from fermented grapes. Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different varieties of grapes and strains of yeasts are m ...
making, and is added to
carbonated beverage
A soft drink (see § Terminology for other names) is a drink that usually contains water (often carbonated), a sweetener, and a natural and/or artificial flavoring. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a sugar ...
s like
seltzer
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is water containing dissolved carbon dioxide gas, ...
and beer for effervescence. It has a sharp and acidic odor and generates the taste of
soda water
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is water containing dissolved carbon dioxide gas, ...
in the mouth, but at normally encountered concentrations it is odorless.
[
]
Chemical and physical properties
Structure, bonding and molecular vibrations
The symmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
of a carbon dioxide molecule is linear and centrosymmetric
In crystallography, a centrosymmetric point group contains an inversion center as one of its symmetry elements. In such a point group, for every point (x, y, z) in the unit cell there is an indistinguishable point (-x, -y, -z). Such point groups ...
at its equilibrium geometry. The length
Length is a measure of distance. In the International System of Quantities, length is a quantity with dimension distance. In most systems of measurement a base unit for length is chosen, from which all other units are derived. In the Interna ...
of the carbon-oxygen bond in carbon dioxide is 116.3 pm, noticeably shorter than the roughly 140-pm length of a typical single C–O bond, and shorter than most other C–O multiply-bonded functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s such as carbonyls.[ Since it is centrosymmetric, the molecule has no ]electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
.
 As a linear triatomic molecule, has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at
As a linear triatomic molecule, has four vibrational modes as shown in the diagram. In the symmetric and the antisymmetric stretching modes, the atoms move along the axis of the molecule. There are two bending modes, which are degenerate, meaning that they have the same frequency and same energy, because of the symmetry of the molecule. When a molecule touches a surface or touches another molecule, the two bending modes can differ in frequency because the interaction is different for the two modes. Some of the vibrational modes are observed in the infrared (IR) spectrum: the antisymmetric stretching mode at wavenumber
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temp ...
2349 cm−1 (wavelength 4.25 μm) and the degenerate pair of bending modes at 667 cm−1 (wavelength 15 μm). The symmetric stretching mode does not create an electric dipole so is not observed in IR spectroscopy, but it is detected in by Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman sp ...
at 1388 cm−1 (wavelength 7.2 μm).
In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure. However, in a Coulomb explosion imaging experiment, an instantaneous image of the molecular structure can be deduced. Such an experiment has been performed for carbon dioxide.
The result of this experiment, and the conclusion of theoretical calculations based on an ab initio potential energy surface of the molecule, is that none of the molecules in the gas phase are ever exactly linear.
In aqueous solution
Carbon dioxide is soluble in water, in which it reversibly forms (carbonic acid), which is a weak acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a hydron (chemistry), proton, H+, and an anion, A-. The Dissociation (chemistry), dissociation of a strong acid in solution is effectively comple ...
since its ionization in water is incomplete.
:CO2 + H2O <=> H2CO3
The hydration equilibrium constant of carbonic acid is, at 25 °C:
:
Hence, the majority of the carbon dioxide is not converted into carbonic acid, but remains as molecules, not affecting the pH.
The relative concentrations of , , and the deprotonated
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu ...
forms (bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
) and (carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
) depend on the pH. As shown in a Bjerrum plot
A Bjerrum plot (named after Niels Bjerrum; sometimes also known as a Sillén diagram or a Hägg diagram) is a graph of the concentrations of the different species of a polyprotic acid in a solution, as a function of pH, when the solution is at ...
, in neutral or slightly alkaline water (pH > 6.5), the bicarbonate form predominates (>50%) becoming the most prevalent (>95%) at the pH of seawater. In very alkaline water (pH > 10.4), the predominant (>50%) form is carbonate. The oceans, being mildly alkaline with typical pH = 8.2–8.5, contain about 120 mg of bicarbonate per liter.
Being diprotic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
, carbonic acid has two acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
s, the first one for the dissociation into the bicarbonate (also called hydrogen carbonate) ion ():
:H2CO3 <=> HCO3- + H+
:''K''a1 = ; p''K''a1 = 3.6 at 25 °C.carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
ion ():
:HCO3- <=> CO3^2- + H+
:''K''a2 = ; p''K''a2 = 10.329
In organisms carbonic acid production is catalysed by the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
, carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site ...
.
Chemical reactions of CO2
is a potent electrophile having an electrophilic reactivity that is comparable to benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
or strong α,β-unsaturated carbonyl compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with are thermodynamically less favored and are often found to be highly reversible. Only very strong nucleophiles, like the carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
s provided by Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
s and organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s react with to give carboxylates:
:MR + CO2 -> RCO2M
:where M = Li or Mg Br and R = alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
.
In metal carbon dioxide complex Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transit ...
es, serves as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
, which can facilitate the conversion of to other chemicals.
The reduction of to CO is ordinarily a difficult and slow reaction:
:CO2 + 2 e- + 2H+ -> CO + H2O
Photoautotrophs Photoautotrophs are organisms that use light energy and inorganic carbon to produce organic materials. Eukaryotic photoautotrophs absorb energy through the chlorophyll molecules in their chloroplasts while prokaryotic photoautotrophs use chlorophyll ...
(i.e. plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclud ...
s and cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blu ...
) use the energy contained in sunlight to photosynthesize
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in c ...
simple sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double ...
s from absorbed from the air and water:
: \mathitCO2 + \mathitH2O -> (CH2O)_\mathit + \mathitO2
The redox potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
for this reaction near pH 7 is about −0.53 V ''versus'' the standard hydrogen electrode. The nickel-containing enzyme carbon monoxide dehydrogenase
In enzymology, carbon monoxide dehydrogenase (CODH) () is an enzyme that catalyzes the chemical reaction
:CO + H2O + A \rightleftharpoons CO2 + AH2
The chemical process catalyzed by carbon monoxide dehydrogenase is similar to the water-gas shif ...
catalyses this process.
Physical properties
 Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.
Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor.standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
, the density of carbon dioxide is around 1.98 kg/m3, about 1.53 times that of air.
Carbon dioxide has no liquid state at pressures below sublimes
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point i ...
directly to a gas above this temperature. In its solid state, carbon dioxide is commonly called dry ice.

Liquid carbon dioxide
Liquid carbon dioxide is the liquid state of carbon dioxide (), which cannot occur under atmospheric pressure. It can only exist at a pressure above , under (temperature of critical point) and above (temperature of triple point). Low-temperatu ...
forms only at pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
s above triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
of carbon dioxide is amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek ''a'' ("wi ...
glass-like solid. This form of glass, called '' carbonia'', is produced by supercooling heated at extreme pressures (40–48 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
, or about 400,000 atmospheres) in a diamond anvil
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It enables the compression of a small (sub-millimeter-sized) piece of material to extreme pressures, typically up to around 10 ...
. This discovery confirmed the theory that carbon dioxide could exist in a glass state similar to other members of its elemental family, like silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
(silica glass) and germanium dioxide. Unlike silica and germania glasses, however, carbonia glass is not stable at normal pressures and reverts to gas when pressure is released.
At temperatures and pressures above the critical point, carbon dioxide behaves as a supercritical fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous so ...
known as supercritical carbon dioxide
Supercritical carbon dioxide (s) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as ...
.
Table of thermal and physical properties of saturated liquid carbon dioxide:
Biological role
Carbon dioxide is an end product of cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
in organisms that obtain energy by breaking down sugars, fats and amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s with oxygen as part of their metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
. This includes all plants, algae and animals and aerobic fungi and bacteria. In vertebrate
Vertebrates () comprise all animal taxa within the subphylum Vertebrata () ( chordates with backbones), including all mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, ...
s, the carbon dioxide travels in the blood from the body's tissues to the skin (e.g., amphibian
Amphibians are tetrapod, four-limbed and ectothermic vertebrates of the Class (biology), class Amphibia. All living amphibians belong to the group Lissamphibia. They inhabit a wide variety of habitats, with most species living within terres ...
s) or the gills (e.g., fish
Fish are aquatic, craniate, gill-bearing animals that lack limbs with digits. Included in this definition are the living hagfish, lampreys, and cartilaginous and bony fish as well as various extinct related groups. Approximately 95% of li ...
), from where it dissolves in the water, or to the lungs from where it is exhaled. During active photosynthesis, plants can absorb more carbon dioxide from the atmosphere than they release in respiration.
Photosynthesis and carbon fixation

Carbon fixation
Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon (particularly in the form of carbon dioxide) is converted to organic compounds by living organisms. The compounds are then used to store energy and as ...
is a biochemical process by which atmospheric carbon dioxide is incorporated by plants, algae and (cyanobacteria) into energy-rich organic molecules such as glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
, thus creating their own food by photosynthesis. Photosynthesis uses carbon dioxide and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
to produce sugars from which other organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s can be constructed, and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
is produced as a by-product.
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly abbreviated to RuBisCO, is the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
involved in the first major step of carbon fixation, the production of two molecules of 3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. Th ...
from CO2 and ribulose bisphosphate
Ribulose 1,5-bisphosphate (RuBP) is an organic substance that is involved in photosynthesis, notably as the principal acceptor in plants. It is a colourless anion, a double phosphate ester of the ketopentose (ketone-containing sugar with five car ...
, as shown in the diagram at left.
RuBisCO is thought to be the single most abundant protein on Earth.
Phototroph
Phototrophs () are organisms that carry out photon capture to produce complex organic compounds (e.g. carbohydrates) and acquire energy. They use the energy from light to carry out various cellular metabolic processes. It is a common misconcep ...
s use the products of their photosynthesis as internal food sources and as raw material for the biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
of more complex organic molecules, such as polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
s, nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s and proteins. These are used for their own growth, and also as the basis of the food chains and webs that feed other organisms, including animals such as ourselves. Some important phototrophs, the coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s synthesise hard calcium carbonate scales. A globally significant species of coccolithophore is ''Emiliania huxleyi
''Emiliania huxleyi'' is a species of coccolithophore found in almost all ocean ecosystems from the equator to sub-polar regions, and from nutrient rich upwelling zones to nutrient poor oligotrophic waters. It is one of thousands of different ...
'' whose calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
scales have formed the basis of many sedimentary rock
Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles ...
s such as limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms whe ...
, where what was previously atmospheric carbon can remain fixed for geological timescales.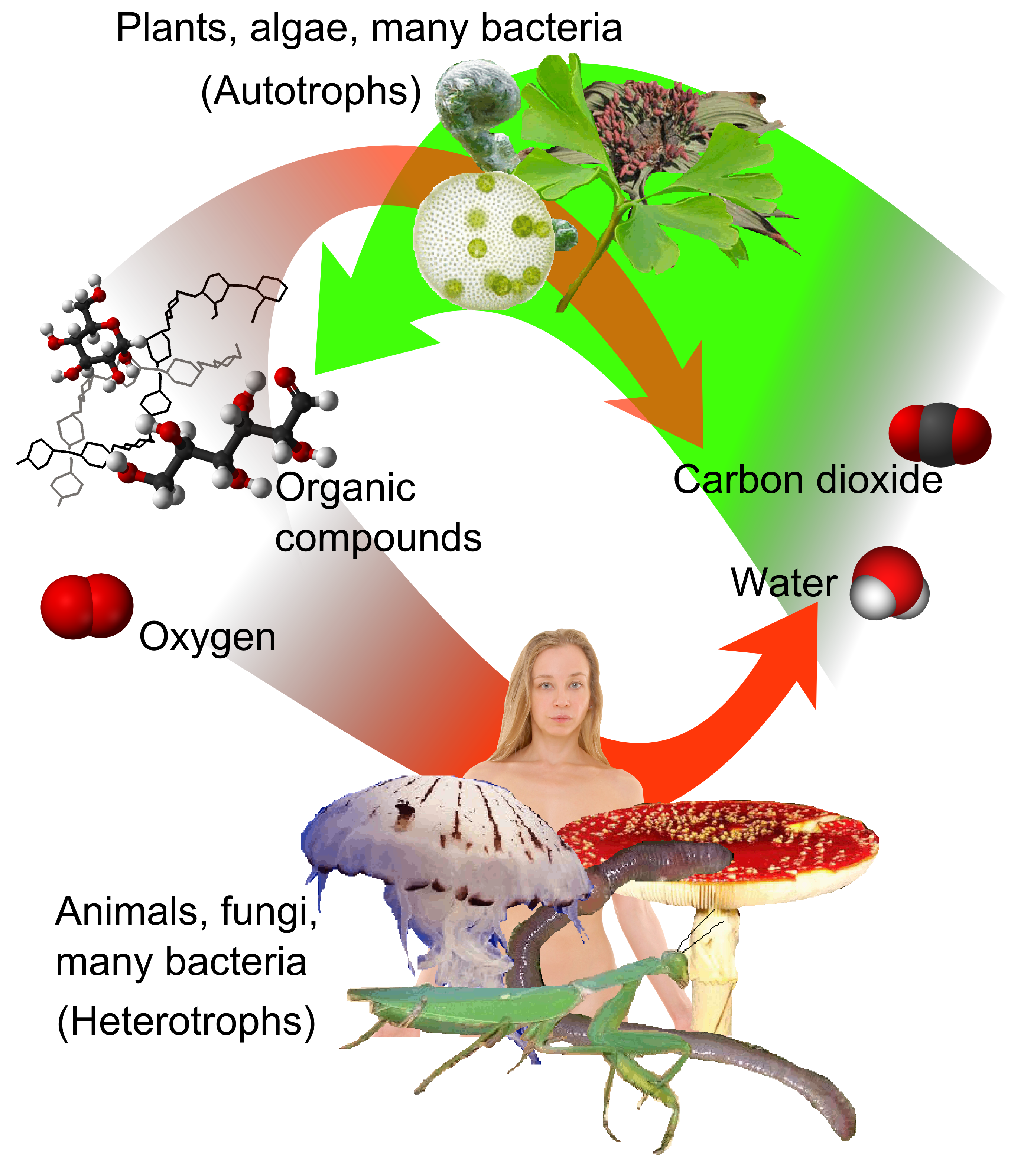 Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased
Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased water-use efficiency
Water-use efficiency (WUE) refers to the ratio of water used in plant metabolism to water lost by the plant through transpiration. Two types of water-use efficiency are referred to most frequently:
* photosynthetic water-use efficiency (also cal ...
. Studies using FACE have shown that CO2 enrichment leads to decreased concentrations of micronutrients in crop plants. This may have knock-on effects on other parts of ecosystem
An ecosystem (or ecological system) consists of all the organisms and the physical environment with which they interact. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy enters the syste ...
s as herbivores will need to eat more food to gain the same amount of protein.
The concentration of secondary metabolites such as phenylpropanoids and flavonoids
can also be altered in plants exposed to high concentrations of CO2.
Plants also emit CO2 during respiration, and so the majority of plants and algae, which use C3 photosynthesis
carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, along with and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phospho ...
, are only net absorbers during the day. Though a growing forest will absorb many tons of CO2 each year, a mature forest will produce as much CO2 from respiration and decomposition of dead specimens (e.g., fallen branches) as is used in photosynthesis in growing plants. Contrary to the long-standing view that they are carbon neutral, mature forests can continue to accumulate carbon and remain valuable carbon sinks, helping to maintain the carbon balance of Earth's atmosphere. Additionally, and crucially to life on earth, photosynthesis by phytoplankton consumes dissolved CO2 in the upper ocean and thereby promotes the absorption of CO2 from the atmosphere.
Toxicity
 Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.
CO2 is an asphyxiant gas and not classified as toxic or harmful in accordance with Globally Harmonized System of Classification and Labelling of Chemicals standards of
Carbon dioxide content in fresh air (averaged between sea-level and 10 kPa level, i.e., about altitude) varies between 0.036% (360 ppm) and 0.041% (412 ppm), depending on the location.
CO2 is an asphyxiant gas and not classified as toxic or harmful in accordance with Globally Harmonized System of Classification and Labelling of Chemicals standards of United Nations Economic Commission for Europe
The United Nations Economic Commission for Europe (ECE or UNECE) is one of the five regional commissions under the jurisdiction of the United Nations Economic and Social Council. It was established in order to promote economic cooperation and i ...
by using the OECD Guidelines for the Testing of Chemicals OECD Guidelines for the Testing of Chemicals (OECD TG) are a set of internationally accepted specifications for the testing of chemicals decided on by the Organisation for Economic Co-operation and Development (OECD). They were first published in ...
. In concentrations up to 1% (10,000 ppm), it will make some people feel drowsy and give the lungs a stuffy feeling.hypercapnia
Hypercapnia (from the Greek ''hyper'' = "above" or "too much" and ''kapnos'' = "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous pro ...
, a subset of asphyxiation
Asphyxia or asphyxiation is a condition of deficient supply of oxygen to the body which arises from abnormal breathing. Asphyxia causes generalized hypoxia, which affects primarily the tissues and organs. There are many circumstances that can i ...
.
Because it is heavier than air, in locations where the gas seeps from the ground (due to sub-surface volcanic or geothermal activity) in relatively high concentrations, without the dispersing effects of wind, it can collect in sheltered/pocketed locations below average ground level, causing animals located therein to be suffocated. Carrion feeders attracted to the carcasses are then also killed. Children have been killed in the same way near the city of Goma
Goma is the capital of North Kivu province in the eastern Democratic Republic of the Congo. It is located on the northern shore of Lake Kivu, next to the Rwandan city of Gisenyi. The lake and the two cities are in the Albertine Rift, the weste ...
by CO2 emissions from the nearby volcano Mount Nyiragongo. The Swahili term for this phenomenon is .
 Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification (
Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification (acidosis
Acidosis is a process causing increased acidity in the blood and other body tissues (i.e., an increase in hydrogen ion concentration). If not further qualified, it usually refers to acidity of the blood plasma.
The term ''acidemia'' describes t ...
). Several studies suggested that 2.0 percent inspired concentrations could be used for closed air spaces (e.g. a submarine
A submarine (or sub) is a watercraft capable of independent operation underwater. It differs from a submersible, which has more limited underwater capability. The term is also sometimes used historically or colloquially to refer to remotely op ...
) since the adaptation is physiological and reversible, as deterioration in performance or in normal physical activity does not happen at this level of exposure for five days. Yet, other studies show a decrease in cognitive function even at much lower levels.
Below 1%
There are few studies of the health effects of long-term continuous CO2 exposure on humans and animals at levels below 1%. Occupational CO2 exposure limits have been set in the United States at 0.5% (5000 ppm) for an eight-hour period. At this CO2 concentration, International Space Station
The International Space Station (ISS) is the largest modular space station currently in low Earth orbit. It is a multinational collaborative project involving five participating space agencies: NASA (United States), Roscosmos (Russia), JAXA ...
crew experienced headaches, lethargy, mental slowness, emotional irritation, and sleep disruption. Studies in animals at 0.5% CO2 have demonstrated kidney calcification and bone loss after eight weeks of exposure. A study of humans exposed in 2.5 hour sessions demonstrated significant negative effects on cognitive abilities at concentrations as low as 0.1% (1000ppm) CO2 likely due to CO2 induced increases in cerebral blood flow.
Ventilation
 Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher CO2 concentrations are associated with occupant health, comfort and performance degradation.
Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher CO2 concentrations are associated with occupant health, comfort and performance degradation. ASHRAE
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, ventilation, air conditioning and refrigeration (HVAC&R) systems design and constructio ...
Standard 62.1–2007 ventilation rates may result in indoor concentrations up to 2,100 ppm above ambient outdoor conditions. Thus if the outdoor concentration is 400 ppm, indoor concentrations may reach 2,500 ppm with ventilation rates that meet this industry consensus standard. Concentrations in poorly ventilated spaces can be found even higher than this (range of 3,000 or 4,000 ppm).
Miners, who are particularly vulnerable to gas exposure due to insufficient ventilation, referred to mixtures of carbon dioxide and nitrogen as " blackdamp," "choke damp" or "stythe." Before more effective technologies were developed, miners would frequently monitor for dangerous levels of blackdamp and other gases in mine shafts by bringing a caged canary
Canary originally referred to the island of Gran Canaria on the west coast of Africa, and the group of surrounding islands (the Canary Islands). It may also refer to:
Animals Birds
* Canaries, birds in the genera ''Serinus'' and ''Crithagra'' i ...
with them as they worked. The canary is more sensitive to asphyxiant gases than humans, and as it became unconscious would stop singing and fall off its perch. The Davy lamp
The Davy lamp is a safety lamp for use in flammable atmospheres, invented in 1815 by Sir Humphry Davy.[methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...](_blank)
, another suffocating gas and explosion risk, would make the lamp burn more brightly.
In February 2020, three people died from suffocation at a party in Moscow when dry ice (frozen CO2) was added to a swimming pool to cool it down. A similar accident occurred in 2018 when a woman died from CO2 fumes emanating from the large amount of dry ice she was transporting in her car.
Outdoor areas with elevated concentrations
Local concentrations of carbon dioxide can reach high values near strong sources, especially those that are isolated by surrounding terrain. At the Bossoleto hot spring near Rapolano Terme
Rapolano Terme is a ''comune'' (municipality) in the Province of Siena in the Italian region Tuscany, located about southeast of Florence and about east of Siena in the area known as the Crete Senesi.
Until 1949 it was known simply as Rapolano.
...
in Tuscany
Tuscany ( ; it, Toscana ) is a Regions of Italy, region in central Italy with an area of about and a population of about 3.8 million inhabitants. The regional capital is Florence (''Firenze'').
Tuscany is known for its landscapes, history, art ...
, Italy, situated in a bowl-shaped depression about in diameter, concentrations of CO2 rise to above 75% overnight, sufficient to kill insects and small animals. After sunrise the gas is dispersed by convection. High concentrations of CO2 produced by disturbance of deep lake water saturated with CO2 are thought to have caused 37 fatalities at Lake Monoun, Cameroon
Cameroon (; french: Cameroun, ff, Kamerun), officially the Republic of Cameroon (french: République du Cameroun, links=no), is a country in west-central Africa. It is bordered by Nigeria to the west and north; Chad to the northeast; the C ...
in 1984 and 1700 casualties at Lake Nyos, Cameroon in 1986.
Human physiology
Content
The body produces approximately of carbon dioxide per day per person, containing of carbon. In humans, this carbon dioxide is carried through the venous system
Veins are blood vessels in humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are the pulmonary and umbilical veins, both of which carry oxygenated b ...
and is breathed out through the lungs, resulting in lower concentrations in the arteries. The carbon dioxide content of the blood is often given as the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
, which is the pressure which carbon dioxide would have had if it alone occupied the volume. In humans, the blood carbon dioxide contents is shown in the adjacent table.
Transport in the blood
CO2 is carried in blood in three different ways. (Exact percentages vary between arterial and venous blood).
* Majority (about 70% to 80%) is converted to bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
ions by the enzyme carbonic anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site ...
in the red blood cells,blood plasma
Blood plasma is a light amber-colored liquid component of blood in which blood cells are absent, but contains proteins and other constituents of whole blood in suspension. It makes up about 55% of the body's total blood volume. It is the intra ...
hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
as carbamino
Carbamino refers to an adduct generated by the addition of carbon dioxide to the free amino group of an amino acid or a protein, such as hemoglobin forming carbaminohemoglobin.
Determining quantity of carboamino in products
It is possible to det ...
compoundsHemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
, the main oxygen-carrying molecule in red blood cell
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
s, carries both oxygen and carbon dioxide. However, the CO2 bound to hemoglobin does not bind to the same site as oxygen. Instead, it combines with the N-terminal groups on the four globin chains. However, because of allosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric site ...
effects on the hemoglobin molecule, the binding of CO2 decreases the amount of oxygen that is bound for a given partial pressure of oxygen. This is known as the Haldane Effect The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood ...
, and is important in the transport of carbon dioxide from the tissues to the lungs. Conversely, a rise in the partial pressure of CO2 or a lower pH will cause offloading of oxygen from hemoglobin, which is known as the Bohr effect
The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration o ...
.
Regulation of respiration
Carbon dioxide is one of the mediators of local autoregulation
Autoregulation is a process within many biological systems, resulting from an internal adaptive mechanism that works to adjust (or mitigate) that system's response to stimuli. While most systems of the body show some degree of autoregulation, it ...
of blood supply. If its concentration is high, the capillaries expand to allow a greater blood flow to that tissue.
Bicarbonate ions are crucial for regulating blood pH. A person's breathing rate influences the level of CO2 in their blood. Breathing that is too slow or shallow causes respiratory acidosis
Respiratory acidosis is a state in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH (a condition generally called acidosis).
Carbon dioxide is produced continuou ...
, while breathing that is too rapid leads to hyperventilation
Hyperventilation is irregular breathing that occurs when the rate or tidal volume of breathing eliminates more carbon dioxide than the body can produce. This leads to hypocapnia, a reduced concentration of carbon dioxide dissolved in the blood. ...
, which can cause respiratory alkalosis.
Although the body requires oxygen for metabolism, low oxygen levels normally do not stimulate breathing. Rather, breathing is stimulated by higher carbon dioxide levels. As a result, breathing low-pressure air or a gas mixture with no oxygen at all (such as pure nitrogen) can lead to loss of consciousness without ever experiencing air hunger. This is especially perilous for high-altitude fighter pilots. It is also why flight attendants instruct passengers, in case of loss of cabin pressure, to apply the oxygen mask
An oxygen mask provides a method to transfer breathing oxygen gas from a storage tank to the lungs. Oxygen masks may cover only the nose and mouth (oral nasal mask) or the entire face (full-face mask). They may be made of plastic, silicone, or r ...
to themselves first before helping others; otherwise, one risks losing consciousness.
Concentrations and role in the environment
Atmosphere

Oceans
Ocean acidification
Carbon dioxide dissolves in the ocean to form carbonic acid (H2CO3), bicarbonate (HCO3−) and carbonate (CO32−). There is about fifty times as much carbon dioxide dissolved in the oceans as exists in the atmosphere. The oceans act as an enormous carbon sink, and have taken up about a third of CO2 emitted by human activity.

Hydrothermal vents
Carbon dioxide is also introduced into the oceans through hydrothermal vents. The ''Champagne'' hydrothermal vent, found at the Northwest Eifuku volcano in the Mariana Trench, produces almost pure liquid carbon dioxide, one of only two known sites in the world as of 2004, the other being in the Okinawa Trough
The (also called , literally China-Ryukyu Border Trough ) is a seabed feature of the East China Sea. It is an active, initial back-arc rifting basin which has formed behind the Ryukyu arc-trench system in the West Pacific. It developed where th ...
. The finding of a submarine lake of liquid carbon dioxide in the Okinawa Trough was reported in 2006.
Production
Biological processes
Carbon dioxide is a by-product of the fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
of sugar in the brewing
Brewing is the production of beer by steeping a starch source (commonly cereal grains, the most popular of which is barley) in water and #Fermenting, fermenting the resulting sweet liquid with Yeast#Beer, yeast. It may be done in a brewery ...
of beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
, whisky and other alcoholic beverage
An alcoholic beverage (also called an alcoholic drink, adult beverage, or a drink) is a drink that contains ethanol, a type of alcohol that acts as a drug and is produced by fermentation of grains, fruits, or other sources of sugar. The c ...
s and in the production of bioethanol. Yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
metabolizes sugar to produce and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
, also known as alcohol, as follows:
: C6H12O6 -> 2 CO2 + 2 C2H5OH
All aerobic organisms produce when they oxidize carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s, fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, fr ...
s, and protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s. The large number of reactions involved are exceedingly complex and not described easily. Refer to (cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
, anaerobic respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing re ...
and photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
). The equation for the respiration of glucose and other monosaccharide
Monosaccharides (from Greek ''monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
They are usually colorless, water-solub ...
s is:
: C6H12O6 + 6 O2 -> 6 CO2 + 6 H2O
Anaerobic organisms
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenate ...
decompose organic material producing methane and carbon dioxide together with traces of other compounds. Regardless of the type of organic material, the production of gases follows well defined kinetic pattern. Carbon dioxide comprises about 40–45% of the gas that emanates from decomposition in landfills (termed "landfill gas
Landfill gas is a mix of different gases created by the action of microorganisms within a landfill as they decompose organic waste, including for example, food waste and paper waste. Landfill gas is approximately forty to sixty percent methane, ...
"). Most of the remaining 50–55% is methane.
Industrial processes
Carbon dioxide can be obtained by distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
from air, but the method is inefficient. Industrially, carbon dioxide is predominantly an unrecovered waste product, produced by several methods which may be practiced at various scales.
Combustion
The combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
of all carbon-based fuels, such as methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
(natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
), petroleum distillates (gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic co ...
, diesel
Diesel may refer to:
* Diesel engine, an internal combustion engine where ignition is caused by compression
* Diesel fuel, a liquid fuel used in diesel engines
* Diesel locomotive, a railway locomotive in which the prime mover is a diesel engin ...
, kerosene
Kerosene, paraffin, or lamp oil is a combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in aviation as well as households. Its name derives from el, κηρός (''keros'') meaning "wax", and was regi ...
, propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used a ...
), coal, wood and generic organic matter produces carbon dioxide and, except in the case of pure carbon, water. As an example, the chemical reaction between methane and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
:
: CH4 + 2 O2-> CO2 + 2 H2O
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
is reduced from its oxides with coke in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric ...
, producing pig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
and carbon dioxide:
By-product from hydrogen production
Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming and the water gas shift reaction in ammonia production
Ammonia is one of the most highly produced inorganic chemicals. There are numerous large-scale ammonia plants worldwide, producing a grand total of 144 million tonnes of nitrogen (equivalent to 175 million tonnes of ammonia) in 2016. This has incr ...
. These processes begin with the reaction of water and natural gas (mainly methane). This is a major source of food-grade carbon dioxide for use in carbonation of beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
and soft drink
A soft drink (see § Terminology for other names) is a drink that usually contains water (often carbonated), a sweetener, and a natural and/or artificial flavoring. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a su ...
s, and is also used for stunning animals such as poultry
Poultry () are domesticated birds kept by humans for their eggs, their meat or their feathers. These birds are most typically members of the superorder Galloanserae (fowl), especially the order Galliformes (which includes chickens, quails, a ...
. In the summer of 2018 a shortage of carbon dioxide for these purposes arose in Europe due to the temporary shut-down of several ammonia plants for maintenance.
Thermal decomposition of limestone
It is produced by thermal decomposition of limestone, by heating ( calcining) at about , in the manufacture of quicklime
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic ma ...
(calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" co ...
, ), a compound that has many industrial uses:
: CaCO3 -> CaO + CO2
Acids liberate from most metal carbonates. Consequently, it may be obtained directly from natural carbon dioxide springs, where it is produced by the action of acidified water on limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms whe ...
or dolomite Dolomite may refer to:
*Dolomite (mineral), a carbonate mineral
*Dolomite (rock), also known as dolostone, a sedimentary carbonate rock
*Dolomite, Alabama, United States, an unincorporated community
*Dolomite, California, United States, an unincor ...
. The reaction between hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and calcium carbonate (limestone or chalk) is shown below:
:CaCO3 + 2HCl -> CaCl2 + H2CO3
The carbonic acid () then decomposes to water and :
:H2CO3 -> CO2 + H2O
Such reactions are accompanied by foaming or bubbling, or both, as the gas is released. They have widespread uses in industry because they can be used to neutralize waste acid streams.
Commercial uses
Carbon dioxide is used by the food industry, the oil industry, and the chemical industry.
Precursor to chemicals
In the chemical industry, carbon dioxide is mainly consumed as an ingredient in the production of urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
, with a smaller fraction being used to produce methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
and a range of other products. Some carboxylic acid derivatives such as sodium salicylate are prepared using CO2 by the Kolbe-Schmitt reaction.
In addition to conventional processes using CO2 for chemical production, electrochemical methods are also being explored at a research level. In particular, the use of renewable energy for production of fuels from CO2 (such as methanol) is attractive as this could result in fuels that could be easily transported and used within conventional combustion technologies but have no net CO2 emissions.
Agriculture
Plants require carbon dioxide to conduct photosynthesis. The atmospheres of greenhouses may (if of large size, must) be enriched with additional CO2 to sustain and increase the rate of plant growth. At very high concentrations (100 times atmospheric concentration, or greater), carbon dioxide can be toxic to animal life, so raising the concentration to 10,000 ppm (1%) or higher for several hours will eliminate pests such as whiteflies
Whiteflies are Hemipterans that typically feed on the undersides of plant leaves. They comprise the family Aleyrodidae, the only family in the superfamily Aleyrodoidea. More than 1550 species have been described.
Description and taxonomy
The ...
and spider mite
Spider mites are members of the Tetranychidae family, which includes about 1,200 species. They are part of the subclass Acari (mites). Spider mites generally live on the undersides of leaves of plants, where they may spin protective silk webs, a ...
s in a greenhouse.
Foods
 Carbon dioxide is a
Carbon dioxide is a food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salt ...
used as a propellant and acidity regulator in the food industry. It is approved for usage in the EU (listed as E number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
E290), US and Australia and New Zealand (listed by its INS number 290).
A candy called Pop Rocks
Pop Rocks, also called popping candy, is a candy, owned by Zeta Espacial S.A. Pop Rocks ingredients include sugar, lactose (milk sugar), and flavoring. It differs from typical hard candy in that pressurized carbon dioxide gas bubbles are emb ...
is pressurized with carbon dioxide gas at about . When placed in the mouth, it dissolves (just like other hard candy) and releases the gas bubbles with an audible pop.
Leavening agents cause dough to rise by producing carbon dioxide. Baker's yeast produces carbon dioxide by fermentation of sugars within the dough, while chemical leaveners such as baking powder and baking soda release carbon dioxide when heated or if exposed to acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s.
Beverages
Carbon dioxide is used to produce carbonated soft drink
A soft drink (see § Terminology for other names) is a drink that usually contains water (often carbonated), a sweetener, and a natural and/or artificial flavoring. The sweetener may be a sugar, high-fructose corn syrup, fruit juice, a su ...
s and soda water
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is water containing dissolved carbon dioxide gas, ...
. Traditionally, the carbonation of beer and sparkling wine came about through natural fermentation, but many manufacturers carbonate these drinks with carbon dioxide recovered from the fermentation process. In the case of bottled and kegged beer, the most common method used is carbonation with recycled carbon dioxide. With the exception of British real ale, draught beer is usually transferred from kegs in a cold room or cellar to dispensing taps on the bar using pressurized carbon dioxide, sometimes mixed with nitrogen.
The taste of soda water (and related taste sensations in other carbonated beverages) is an effect of the dissolved carbon dioxide rather than the bursting bubbles of the gas. Carbonic anhydrase 4 converts to carbonic acid leading to a sour taste, and also the dissolved carbon dioxide induces a somatosensory
In physiology, the somatosensory system is the network of neural structures in the brain and body that produce the perception of touch (haptic perception), as well as temperature (thermoception), body position (proprioception), and pain. It is ...
response.
Winemaking
 Carbon dioxide in the form of dry ice is often used during the
Carbon dioxide in the form of dry ice is often used during the cold soak
Cold is the presence of low temperature, especially in the atmosphere. In common usage, cold is often a subjective perception. A lower bound to temperature is absolute zero, defined as 0.00K on the Kelvin scale, an absolute thermodynamic ...
phase in winemaking
Winemaking or vinification is the production of wine, starting with the selection of the fruit, its fermentation into alcohol, and the bottling of the finished liquid. The history of wine-making stretches over millennia. The science of wine and ...
to cool clusters of grape
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus ''Vitis''. Grapes are a non- climacteric type of fruit, generally occurring in clusters.
The cultivation of grapes began perhaps 8,000 years ago, ...
s quickly after picking to help prevent spontaneous fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
by wild yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitut ...
. The main advantage of using dry ice over water ice is that it cools the grapes without adding any additional water that might decrease the sugar concentration in the grape must
Must (from the Latin ''vinum mustum'', "young wine") is freshly crushed fruit juice (usually grape juice) that contains the skins, seeds, and stems of the fruit. The solid portion of the must is called pomace and typically makes up 7–23% of th ...
, and thus the alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
concentration in the finished wine. Carbon dioxide is also used to create a hypoxic environment for carbonic maceration, the process used to produce Beaujolais wine.
Carbon dioxide is sometimes used to top up wine bottles or other storage
Storage may refer to:
Goods Containers
* Dry cask storage, for storing high-level radioactive waste
* Food storage
* Intermodal container, cargo shipping
* Storage tank
Facilities
* Garage (residential), a storage space normally used to store car ...
vessels such as barrels to prevent oxidation, though it has the problem that it can dissolve into the wine, making a previously still wine slightly fizzy. For this reason, other gases such as nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
or argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
are preferred for this process by professional wine makers.
Stunning animals
Carbon dioxide is often used to "stun" animals before slaughter. "Stunning" may be a misnomer, as the animals are not knocked out immediately and may suffer distress.
Inert gas
Carbon dioxide is one of the most commonly used compressed gases for pneumatic (pressurized gas) systems in portable pressure tools. Carbon dioxide is also used as an atmosphere for welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing Fusion welding, fusion. Welding is distinct from lower ...
, although in the welding arc, it reacts to oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle than those made in more inert atmospheres. When used for MIG welding
Russian Aircraft Corporation "MiG" (russian: Российская самолётостроительная корпорация „МиГ“, Rossiyskaya samolyotostroitel'naya korporatsiya "MiG"), commonly known as Mikoyan and MiG, was a Russi ...
, CO2 use is sometimes referred to as MAG welding, for Metal Active Gas, as CO2 can react at these high temperatures. It tends to produce a hotter puddle than truly inert atmospheres, improving the flow characteristics. Although, this may be due to atmospheric reactions occurring at the puddle site. This is usually the opposite of the desired effect when welding, as it tends to embrittle the site, but may not be a problem for general mild steel welding, where ultimate ductility is not a major concern.
Carbon dioxide is used in many consumer products that require pressurized gas because it is inexpensive and nonflammable, and because it undergoes a phase transition from gas to liquid at room temperature at an attainable pressure of approximately , allowing far more carbon dioxide to fit in a given container than otherwise would. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
capsules of CO2 are also sold as supplies of compressed gas for air gun
An air gun or airgun is a gun that fires projectiles pneumatically with compressed air or other gases that are mechanically pressurized ''without'' involving any chemical reactions, in contrast to a firearm, which pressurizes gases ''chemica ...
s, paintball markers/guns, inflating bicycle tires, and for making carbonated water
Carbonated water (also known as soda water, sparkling water, fizzy water, club soda, water with gas, in many places as mineral water, or especially in the United States as seltzer or seltzer water) is water containing dissolved carbon dioxide gas, ...
. High concentrations of carbon dioxide can also be used to kill pests. Liquid carbon dioxide is used in supercritical drying of some food products and technological materials, in the preparation of specimens for scanning electron microscopycoffee bean
A coffee bean is a seed of the ''Coffea'' plant and the source for coffee. It is the pip inside the red or purple fruit often referred to as a coffee cherry. Just like ordinary cherries, the coffee fruit is also a so-called stone fruit. Even thou ...
s.
Fire extinguisher
 Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for electrical fire
A fire class is a system of categorizing fire with regard to the type of material and fuel for combustion. Class letters are often assigned to the different types of fire, but these differ between territories. There are separate standards for t ...
s, contain liquid carbon dioxide under pressure. Carbon dioxide extinguishers work well on small flammable liquid and electrical fires, but not on ordinary combustible fires, because they do not cool the burning substances significantly, and when the carbon dioxide disperses, they can catch fire upon exposure to atmospheric oxygen
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary approach of research and draws on environmental chemistry, physics, meteorol ...
. They are mainly used in server rooms.
Carbon dioxide has also been widely used as an extinguishing agent in fixed fire-protection systems for local application of specific hazards and total flooding of a protected space. International Maritime Organization
The International Maritime Organization (IMO, French: ''Organisation maritime internationale'') is a specialised agency of the United Nations responsible for regulating shipping. The IMO was established following agreement at a UN conference ...
standards recognize carbon-dioxide systems for fire protection of ship holds and engine rooms. Carbon-dioxide-based fire-protection systems have been linked to several deaths, because it can cause suffocation in sufficiently high concentrations. A review of CO2 systems identified 51 incidents between 1975 and the date of the report (2000), causing 72 deaths and 145 injuries.
Supercritical CO2 as solvent
Liquid carbon dioxide is a good solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
for many lipophilic organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s and is used to remove caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
from coffee
Coffee is a drink prepared from roasted coffee beans. Darkly colored, bitter, and slightly acidic, coffee has a stimulant, stimulating effect on humans, primarily due to its caffeine content. It is the most popular hot drink in the world.
S ...
.pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and re ...
and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It is also used by some dry cleaners
Dry cleaning is any cleaning process for clothing and textiles using a solvent other than water.
Dry cleaning still involves liquid, but clothes are instead soaked in a water-free liquid solvent. Tetrachloroethylene (perchloroethylene), known in ...
for this reason. It is used in the preparation of some aerogels
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low ...
because of the properties of supercritical carbon dioxide.
Medical and pharmacological uses
In medicine, up to 5% carbon dioxide (130 times atmospheric concentration) is added to oxygen for stimulation of breathing after apnea
Apnea, BrE: apnoea, is the temporal cessation of breathing. During apnea, there is no movement of the muscles of inhalation, and the volume of the lungs initially remains unchanged. Depending on how blocked the airways are ( patency), there ...
and to stabilize the / balance in blood.
Carbon dioxide can be mixed with up to 50% oxygen, forming an inhalable gas; this is known as Carbogen
Carbogen, also called Meduna's Mixture after its inventor Ladislas J. Meduna, Ladislas Meduna, is a mixture of carbon dioxide and oxygen gas. Meduna's original formula was 30% CO2 and 70% oxygen, but the term carbogen can refer to any mixture of t ...
and has a variety of medical and research uses.
Another medical use are the mofette, dry spas that use carbon dioxide from post-volcanic discharge for therapeutic purposes.
Energy
Supercritical CO2 is used as the working fluid in the Allam power cycle engine.
Fossil fuel recovery
Carbon dioxide is used in enhanced oil recovery
Enhanced oil recovery (abbreviated EOR), also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted otherwise. EOR can extract 30% to 60% or more of a reservoir's oil, compared to 20% to 40% using ...
where it is injected into or adjacent to producing oil wells, usually under supercritical conditions, when it becomes miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
with the oil. This approach can increase original oil recovery by reducing residual oil saturation by between 7% to 23% additional to primary extraction. It acts as both a pressurizing agent and, when dissolved into the underground crude oil
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
, significantly reduces its viscosity, and changing surface chemistry enabling the oil to flow more rapidly through the reservoir to the removal well. In mature oil fields, extensive pipe networks are used to carry the carbon dioxide to the injection points.
In enhanced coal bed methane recovery Enhanced coal bed methane recovery is a method of producing additional coalbed methane from a source rock, similar to enhanced oil recovery applied to oil fields. Carbon dioxide (CO2) injected into a bituminous coal bed would occupy pore space ...
, carbon dioxide would be pumped into the coal seam to displace methane, as opposed to current methods which primarily rely on the removal of water (to reduce pressure) to make the coal seam release its trapped methane.
Bio transformation into fuel
It has been proposed that CO2 from power generation be bubbled into ponds to stimulate growth of algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
that could then be converted into biodiesel
Biodiesel is a form of diesel fuel derived from plants or animals and consisting of long-chain fatty acid esters. It is typically made by chemically reacting lipids such as animal fat (tallow), soybean oil, or some other vegetable oil with ...
fuel.Synechococcus elongatus
''Synechococcus elongatus'' is a unicellular cyanobacterium that has a rapid autotrophic growth comparable to yeast. Its ability to grow rapidly using sunlight has implications for biotechnological applications, especially when incorporating gene ...
'' has been genetically engineered to produce the fuels isobutyraldehyde and isobutanol
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as ''i''-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either dire ...
from CO2 using photosynthesis.
Researchers have developed a process called electrolysis, using enzymes isolated from bacteria to power the chemical reactions which convert CO2 into fuels.
= Refrigerant
=
 Liquid and solid carbon dioxide are important
Liquid and solid carbon dioxide are important refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
s, especially in the food industry, where they are employed during the transportation and storage of ice cream and other frozen foods. Solid carbon dioxide is called "dry ice" and is used for small shipments where refrigeration equipment is not practical. Solid carbon dioxide is always below at regular atmospheric pressure, regardless of the air temperature.
Liquid carbon dioxide (industry nomenclature R744 or R-744) was used as a refrigerant prior to the use of dichlorodifluoromethane
Dichlorodifluoromethane (R-12) is a colorless gas usually sold under the brand name Freon-12, and a chlorofluorocarbon halomethane (CFC) used as a refrigerant and aerosol spray propellant. Complying with the Montreal Protocol, its manufacture was ...
(R12, a chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
(CFC) compound). might enjoy a renaissance because one of the main substitutes to CFCs, 1,1,1,2-tetrafluoroethane
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties s ...
( R134a, a hydrofluorocarbon
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditi ...
(HFC) compound) contributes to climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
more than does. physical properties are highly favorable for cooling, refrigeration, and heating purposes, having a high volumetric cooling capacity. Due to the need to operate at pressures of up to , systems require highly mechanically resistant reservoirs and components that have already been developed for mass production in many sectors. In automobile air conditioning, in more than 90% of all driving conditions for latitudes higher than 50°, (R744) operates more efficiently than systems using HFCs (e.g., R134a). Its environmental advantages ( GWP of 1, non-ozone depleting, non-toxic, non-flammable) could make it the future working fluid to replace current HFCs in cars, supermarkets, and heat pump water heaters, among others. Coca-Cola
Coca-Cola, or Coke, is a carbonated soft drink manufactured by the Coca-Cola Company. Originally marketed as a temperance drink and intended as a patent medicine, it was invented in the late 19th century by John Stith Pemberton in Atlanta ...
has fielded -based beverage coolers and the U.S. Army is interested in refrigeration and heating technology.
Minor uses
 Carbon dioxide is the
Carbon dioxide is the lasing medium
The active laser medium (also called gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from a ...
in a carbon-dioxide laser
The carbon-dioxide laser (CO2 laser) was one of the earliest gas lasers to be developed. It was invented by Kumar Patel of Bell Labs in 1964 and is still one of the most useful types of laser. Carbon-dioxide lasers are the highest-power continuou ...
, which is one of the earliest type of lasers.
Carbon dioxide can be used as a means of controlling the pH of swimming pools, by continuously adding gas to the water, thus keeping the pH from rising. Among the advantages of this is the avoidance of handling (more hazardous) acids. Similarly, it is also used in the maintaining reef aquaria
A reef aquarium or reef tank is a marine aquarium that prominently displays live corals and other marine invertebrates as well as fish that play a role in maintaining the tropical coral reef environment. A reef aquarium requires appropriately ...
, where it is commonly used in calcium reactor __NOTOC__
In marine and reef aquariums, a calcium reactor creates a balance of alkalinity. An acidic solution is produced by injecting carbon dioxide into a chamber with salt water and calcium rich media. The carbon dioxide lowers the pH by produc ...
s to temporarily lower the pH of water being passed over calcium carbonate in order to allow the calcium carbonate to dissolve into the water more freely, where it is used by some coral
Corals are marine invertebrates within the class Anthozoa of the phylum Cnidaria. They typically form compact colonies of many identical individual polyps. Coral species include the important reef builders that inhabit tropical oceans and sec ...
s to build their skeleton.
Used as the primary coolant in the British advanced gas-cooled reactor for nuclear power generation.
Carbon dioxide induction is commonly used for the euthanasia of laboratory research animals. Methods to administer CO2 include placing animals directly into a closed, prefilled chamber containing CO2, or exposure to a gradually increasing concentration of CO2. The American Veterinary Medical Association
The American Veterinary Medical Association (AVMA), founded in 1863, is a not-for-profit association representing more than 99,500 veterinarians in the US.
The AVMA provides information resources, continuing education opportunities, publicatio ...
's 2020 guidelines for carbon dioxide induction state that a displacement rate of 30% to 70% of the chamber or cage volume per minute is optimal for the humane euthanasia of small rodents.
History of discovery
 Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish
Flemish (''Vlaams'') is a Low Franconian dialect cluster of the Dutch language. It is sometimes referred to as Flemish Dutch (), Belgian Dutch ( ), or Southern Dutch (). Flemish is native to Flanders, a historical region in northern Belgium; ...
chemist Jan Baptist van Helmont observed that when he burned charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, cal ...
in a closed vessel, the mass of the resulting ash
Ash or ashes are the solid remnants of fires. Specifically, ''ash'' refers to all non-aqueous, non- gaseous residues that remain after something burns. In analytical chemistry, to analyse the mineral and metal content of chemical samples, ash ...
was much less than that of the original charcoal. His interpretation was that the rest of the charcoal had been transmuted into an invisible substance he termed a "gas" or "wild spirit" (''spiritus sylvestris'').
The properties of carbon dioxide were further studied in the 1750s by the Scottish
Scottish usually refers to something of, from, or related to Scotland, including:
*Scottish Gaelic, a Celtic Goidelic language of the Indo-European language family native to Scotland
*Scottish English
*Scottish national identity, the Scottish ide ...
physician Joseph Black. He found that limestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms whe ...
( calcium carbonate) could be heated or treated with acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s to yield a gas he called "fixed air." He observed that the fixed air was denser than air and supported neither flame nor animal life. Black also found that when bubbled through limewater
Limewater is the common name for a saturated aqueous solution of calcium hydroxide. Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). "Pure" (i.e. less than or fully saturated) limewater i ...
(a saturated aqueous solution of calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has m ...
), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation. In 1772, English chemist Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
published a paper entitled ''Impregnating Water with Fixed Air'' in which he described a process of dripping sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
(or ''oil of vitriol'' as Priestley knew it) on chalk in order to produce carbon dioxide, and forcing the gas to dissolve by agitating a bowl of water in contact with the gas.Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
and Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inducti ...
.Adrien-Jean-Pierre Thilorier Adrien-Jean-Pierre Thilorier (16 February 1790 – 2 December 1844) was a French inventor who was the first person to produce solid carbon dioxide ("dry ice").
Early years
Adrien-Jean-Pierre Thilorier was born in Paris, France, on 16 February 1790 ...
, who in 1835 opened a pressurized container of liquid carbon dioxide, only to find that the cooling produced by the rapid evaporation of the liquid yielded a "snow" of solid CO2.
See also
*
*
* (from the atmosphere)
*
*
*
* List of least carbon efficient power stations
This is a list of least carbon efficient power stations in selected countries. Lists were created by the WWF and lists the most polluting power stations in terms of the level of carbon dioxide produced per unit of electricity generated. In genera ...
* List of countries by carbon dioxide emissions
*
*
* (early work on CO2 and climate change)
*
* NASA's
*
*
References
External links
Current global map of carbon dioxide concentration
* ttps://gml.noaa.gov/ccgg/trends/ Trends in Atmospheric Carbon Dioxide(NOAA)
{{DEFAULTSORT:Carbon Dioxide
Acid anhydrides
Acidic oxides
Coolants
Fire suppression agents
Greenhouse gases
Household chemicals
Inorganic solvents
Laser gain media
Nuclear reactor coolants
Oxocarbons
Propellants
Refrigerants
Gaseous signaling molecules
E-number additives
 Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor. At
Carbon dioxide is colorless. At low concentrations the gas is odorless; however, at sufficiently high concentrations, it has a sharp, acidic odor. At  Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased
Plants can grow as much as 50 percent faster in concentrations of 1,000 ppm CO2 when compared with ambient conditions, though this assumes no change in climate and no limitation on other nutrients. Elevated CO2 levels cause increased growth reflected in the harvestable yield of crops, with wheat, rice and soybean all showing increases in yield of 12–14% under elevated CO2 in FACE experiments.
Increased atmospheric CO2 concentrations result in fewer stomata developing on plants which leads to reduced water usage and increased  Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification (
Adaptation to increased concentrations of CO2 occurs in humans, including modified breathing and kidney bicarbonate production, in order to balance the effects of blood acidification ( Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher CO2 concentrations are associated with occupant health, comfort and performance degradation.
Poor ventilation is one of the main causes of excessive CO2 concentrations in closed spaces. Carbon dioxide differential above outdoor concentrations at steady state conditions (when the occupancy and ventilation system operation are sufficiently long that CO2 concentration has stabilized) are sometimes used to estimate ventilation rates per person. Higher CO2 concentrations are associated with occupant health, comfort and performance degradation. 

 Carbon dioxide is a
Carbon dioxide is a  Carbon dioxide in the form of dry ice is often used during the
Carbon dioxide in the form of dry ice is often used during the  Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for
Carbon dioxide can be used to extinguish flames by flooding the environment around the flame with the gas. It does not itself react to extinguish the flame, but starves the flame of oxygen by displacing it. Some fire extinguishers, especially those designed for  Carbon dioxide is the
Carbon dioxide is the  Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the