Discovery And Development Of Angiotensin Receptor Blockers on:
[Wikipedia]
[Google]
[Amazon]
The
Two more angiotensin receptors have been described, AT3 and AT4, but their role is still unknown.
AT2 receptors are highly expressed in the developing
 Ang II binds to AT1 receptors via various
Ang II binds to AT1 receptors via various
The ionic bridge formed between Lys199 and the carboxyl terminal group of the Phe8 residue of Ang II is most likely stabilized by the Trp253 residue. In addition, Phe259 and Asp263 in transmembrane helix 6 and Lys102 and
 Blood pressure and fluid and electrolyte
Blood pressure and fluid and electrolyte

The 4-carboxy-derivative EXP-6155 had a binding activity which was ten-fold greater than that of S-8308 which further strengthened this
Then the polar carboxyl group was replaced with a more lipophilic
There are three functional groups that are the most important parts for the
The first one is the imidazole ring that binds to amino acids in helix 7 ( Asn295). The second group is the biphenyl-methyl group that binds to amino acids in both helices 6 and 7 ( Phe301, Phe300, Trp253 and
The tetrazole group has been successfully replaced by a carboxylic acid group as is the case with telmisartan. Structure-activity relationship (SAR)
Most of the ARBs have the same
In valsartan, the imidazole ring of losartan has been replaced with an acylated amino acid.
Several substituents have been tried at the 4- and 5- positions on the imidazole ring. The chloro and hydroxymethyl groups connected to these positions in losartan are probably not of much importance in receptor binding since the other ARBs do not possess these functional groups and have comparable or better binding affinities than losartan. Irbesartan has a carbonyl group at the 5-position, functioning as a hydrogen bond acceptor in place of the hydroxymethyl group of losartan, resulting in a longer binding to the receptor.
The structure of eprosartan is the one that differs most from the other ARBs, the usual biphenyl-methyl group has been replaced by a carboxy
Telmisartan has a carboxylic acid at the 2-position of the biphenyl-methyl group and is more potent than the tetrazole analogue.
It has been reported that
It has also been reported that an hydroxy group in the 4-position on the imidazole ring, plays an important role in the binding affinity and compensates for the disadvantage of
These results show that a medium-sized hydroxy alkyl group, such as CHMeOH and CMe2OH, is favorable for the substituent of the 4-position on the imidazole ring. Furthermore, the
 Several new nonpeptide ARBs are undergoing clinical trials or are at pre-clinical stages of development. Among these are embusartan (BAY 10-6734 or BAY 10-6734), KRH-594, fonsartan (HR 720) and pratosartan (KT3-671). Pratosartan, for example, has a novel structure: a seven-membered ring that bears an oxo moiety (C=O) fused to the imidazole ring (figure 4), and its affinity for the AT1 receptor is about 7 times higher than losartan's. The purpose of the oxo group is similar to that of the carboxylic acid groups on other ARBs.
Several new nonpeptide ARBs are undergoing clinical trials or are at pre-clinical stages of development. Among these are embusartan (BAY 10-6734 or BAY 10-6734), KRH-594, fonsartan (HR 720) and pratosartan (KT3-671). Pratosartan, for example, has a novel structure: a seven-membered ring that bears an oxo moiety (C=O) fused to the imidazole ring (figure 4), and its affinity for the AT1 receptor is about 7 times higher than losartan's. The purpose of the oxo group is similar to that of the carboxylic acid groups on other ARBs.
Other attributes of ARBs are also under investigation, such as the positive effects of telmisartan on
angiotensin receptor blockers
Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals tha ...
(ARBs), also called angiotensin (AT1) receptor antagonists or sartans, are a group of antihypertensive
Antihypertensives are a class of drugs that are used to treat hypertension (high blood pressure). Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke and myocardial infarction. Evidence suggests tha ...
drugs that act by blocking the effects of the hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
angiotensin II (Ang II) in the body, thereby lowering blood pressure. Their structure is similar to Ang II and they bind to Ang II receptors as inhibitors, e.g., 24 from Rhys Healthcare
ARBs are widely used drugs in the clinical setting today, their main indications being mild to moderate hypertension, chronic heart failure, secondary stroke prevention and diabetic nephropathy
Diabetic nephropathy, also known as diabetic kidney disease, is the chronic loss of kidney function occurring in those with diabetes mellitus. Diabetic nephropathy is the leading causes of chronic kidney disease (CKD) and end-stage renal disease ...
.
The discovery and development of ARBs is a demonstrative example of modern rational drug design
Drug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activa ...
and how design can be used to gain further knowledge of physiological systems, in this case, the characterization of the subtypes of Ang II receptors.
History
In 1898, the physiologistRobert Tigerstedt
Robert Adolph Armand Tigerstedt (28 February 1853 – 12 February 1923) was a Finnish-born medical scientist and physiologist who, with his student Per Bergman, discovered renin at the Karolinska Institute, Stockholm in 1898. Renin is a compo ...
and his student, Per Bergman, experimented with rabbits by injecting them with kidney extracts. Their results suggested the kidneys produced a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
, which they named renin
Renin (etymology and pronunciation), also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin–angiotensin–aldosterone system (RAAS)—also known as the r ...
, that caused a rise in blood pressure. In the 1930s, Goldblatt conducted experiments where he constricted the renal blood flow in dogs; he found the ischaemic
Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism (to keep tissue alive). Ischemia is generally caused by problems wi ...
kidneys did in fact secrete a chemical that caused vasoconstriction
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vess ...
. In 1939, renin was found not to cause the rise in blood pressure, but was an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
which catalyzed the formation of the substances that were responsible, namely, angiotensin I
Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the a ...
(Ang I) and Ang II.
In the 1970s, scientists first observed Ang II to harm the heart and kidneys, and individuals with high levels of renin activity in plasma were at increased risk of myocardial infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may ...
and stroke.
With the introduction of angiotensin converting enzyme (ACE) inhibitors in the late 1970s it was confirmed that Ang II plays an important role in regulating blood pressure and electrolyte and fluid balance.
Before that attempts had been made to develop useful Ang II receptor antagonists and initially, the main focus was on angiotensin peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
analogues. Saralasin
Saralasin is a competitive angiotensin II receptor antagonist with partial agonistic activity. The aminopeptide sequence for saralasin differs from angiotensin II at three sites:
* At position 1, sarcosine replaces aspartic acid, thereby increasin ...
and other Ang II analogues were potent Ang II receptor blockers but the main problem was a lack of oral bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
.
In the early 1980s it was noted that a series of imidazole-5- acetic acid derivatives diminished blood pressure responses to Ang II in rats. Two compounds, S-8307 and S-8308, were later found to be highly specific and promising non-peptide Ang II receptor antagonists but using molecular modeling
Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials scien ...
it was seen that their structures would have to mimic
MIMIC, known in capitalized form only, is a former simulation computer language developed 1964 by H. E. Petersen, F. J. Sansom and L. M. Warshawsky of Systems Engineering Group within the Air Force Materiel Command at the Wright-Patterson AFB in ...
more closely the pharmacophore
300px, An example of a pharmacophore model.
A pharmacophore is an abstract description of molecular features that are necessary for molecular recognition of a ligand by a biological macromolecule. IUPAC defines a pharmacophore to be "an ensemble o ...
of Ang II. Structural modifications were made and the orally active, potent and selective nonpeptide AT1 receptor blocker losartan
Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besid ...
was developed. In 1995 losartan was approved for clinical use in the United States and since then six additional ARBs have been approved. These drugs are known for their excellent side-effects
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequence ...
profiles, which clinical trials have shown to be similar to those of placebos
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures.
In general, placebos can af ...
.Analogue-based Drug Discovery (O''ptimizing Antihypertensive Therapy by Angiotensin Receptor Blockers''; Farsang, C., Fisher, J., p.157-167) Editors; Fischer, J., Ganellin, R. Wiley-VCH 2006.
The angiotensin II receptor
The actions of Ang II are mediated by angiotensin receptors, AT1 and AT2. These receptors are members of theG protein-coupled receptors
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related p ...
family which are seven transmembrane
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequent ...
helices
A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helices, ...
, connected by interchanging extracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions ...
and intracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions ...
loops.
Each G protein-coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related p ...
couples to a specific G-protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their act ...
which leads to activation of a special effector system. AT1 receptors are for instance primarily coupled through the Gq/11 group of G-proteins
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ac ...
. Two more angiotensin receptors have been described, AT3 and AT4, but their role is still unknown.
Distribution in the body
AT1 receptors are mainly found in the heart,adrenal glands
The adrenal glands (also known as suprarenal glands) are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex whic ...
, brain, liver and kidneys. Their main role is to regulate blood pressure as well as fluid and electrolyte balance. AT2 receptors are highly expressed in the developing
fetus
A fetus or foetus (; plural fetuses, feti, foetuses, or foeti) is the unborn offspring that develops from an animal embryo. Following embryonic development the fetal stage of development takes place. In human prenatal development, fetal dev ...
but they decline rapidly after birth. In the adult, AT2 receptors are present only at low levels and are mostly found in the heart, adrenal glands, uterus, ovaries, kidneys and brain.
Functions
Most of the known actions of Ang II are mediated through the AT1 receptors, for examplevasoconstriction
Vasoconstriction is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vess ...
, aldosterone release, renal sodium reabsorption and vasopressin
Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then trave ...
secretion. The AT2 receptor also takes part in regulation of blood pressure and renal
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; bloo ...
function but mediates antagonistic effects compared to the AT1 receptor.
Binding pockets
 Ang II binds to AT1 receptors via various
Ang II binds to AT1 receptors via various binding sites
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may includ ...
. The primary binding site is at the extracellular region of the AT1 receptor where Ang II interacts with residues in the N-terminus of the AT1 receptor and its first and third extracellular loops. The transmembrane helices also contribute to the binding via the C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
group that interacts with Lys199 in the upper part of helix 5 of the receptor; see figure 1 for details.The ionic bridge formed between Lys199 and the carboxyl terminal group of the Phe8 residue of Ang II is most likely stabilized by the Trp253 residue. In addition, Phe259 and Asp263 in transmembrane helix 6 and Lys102 and
Ser
Ser or SER may refer to:
Places
* Ser, a village in Bogdand Commune, Satu Mare County, Romania
* Serpens (Ser), an astronomical constellation of the northern hemisphere
* Serres, known as Ser in Serbian, a city in Macedonia, Greece
Organization ...
105 in the outer region of transmembrane helix 3 have also been implicated in Ang II binding. This region may possibly participate in the stabilization of the receptor's ratification and in the formation of the intramembrane binding pocket.
Mechanism of action
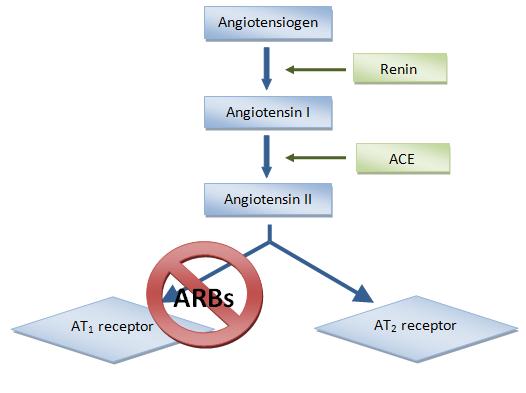
 Blood pressure and fluid and electrolyte
Blood pressure and fluid and electrolyte homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
is regulated by the renin–angiotensin–aldosterone system.
Renin
Renin (etymology and pronunciation), also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin–angiotensin–aldosterone system (RAAS)—also known as the r ...
, an enzyme released from the kidneys, converts the inactive plasma protein angiotensinogen
Angiotensin is a peptide hormone that causes vasoconstriction and an increase in blood pressure. It is part of the renin–angiotensin system, which regulates blood pressure. Angiotensin also stimulates the release of aldosterone from the a ...
into angiotensin I (Ang I). Then Ang I is converted to Ang II with angiotensin converting enzyme
Angiotensin-converting enzyme (), or ACE, is a central component of the renin–angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body. It converts the hormone angiotensin I to the active vasoconstr ...
(ACE), see figure 2. Ang II in plasma then binds to AT-receptors.Goodman & Gilman's The Pharmacological Basis of Therapeutics 11th ed. (''Renin and Angiotensin''; Jackson E.K., 789-821) Editors; Brunton L.L., Lazo J.S., Parker K.L. New York McGraw Hill 2006.
ARBs are blocking the last part of the renin–angiotensin pathway and block the pathway more specifically than ACE inhibitors
Angiotensin-converting-enzyme inhibitors (ACE inhibitors) are a class of medication used primarily for the treatment of high blood pressure and heart failure. They work by causing relaxation of blood vessels as well as a decrease in blood volume ...
.
The AT1 receptor mediates Ang II to cause increased cardiac contractility, sodium reabsorption and vasoconstriction which all lead to increased blood pressure. By blocking AT1 receptors, ARBs lead to lower blood pressure.
An insurmountable inhibition of the AT1 receptor is achieved when the maximum response of Ang II cannot be restored in the presence of the ARB, no matter how high the concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
of Ang II is.
The angiotensin receptor blockers can inhibit the receptor in a competitive surmountable, competitive insurmountable or noncompetitive fashion, depending upon the rate at which they dissociate from the receptor.
Drug discovery and development

Development from saralasin to losartan and eprosartan
For a simple overview of the development of ARBs, see figure 3. Because ofsaralasin
Saralasin is a competitive angiotensin II receptor antagonist with partial agonistic activity. The aminopeptide sequence for saralasin differs from angiotensin II at three sites:
* At position 1, sarcosine replaces aspartic acid, thereby increasin ...
, the first Ang II antagonist, and the development of the first ACE inhibitor captopril, it was generally acknowledged that Ang II receptor antagonists might be promising as effective antihypertensive
Antihypertensives are a class of drugs that are used to treat hypertension (high blood pressure). Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke and myocardial infarction. Evidence suggests tha ...
agents.
Saralasin was developed in the early 1970s and is an octapeptide analogue of Ang II, where the amino acids Asp1, Ile
Ile may refer to:
* iLe, a Puerto Rican singer
* Ile District (disambiguation), multiple places
* Ilé-Ifẹ̀, an ancient Yoruba city in south-western Nigeria
* Interlingue (ISO 639:ile), a planned language
* Isoleucine, an amino acid
* Anothe ...
5 and Phe8 have been replaced with Ser
Ser or SER may refer to:
Places
* Ser, a village in Bogdand Commune, Satu Mare County, Romania
* Serpens (Ser), an astronomical constellation of the northern hemisphere
* Serres, known as Ser in Serbian, a city in Macedonia, Greece
Organization ...
1, Val5 and Ala Ala, ALA, Alaa or Alae may refer to:
Places
* Ala, Hiiu County, Estonia, a village
* Ala, Valga County, Estonia, a village
* Ala, Alappuzha, Kerala, India, a village
* Ala, Iran, a village in Semnan Province
* Ala, Gotland, Sweden
* Alad, S ...
8, respectively. Saralasin was not orally bioavailable
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. Ho ...
, had short duration of action and showed partial agonist
In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonis ...
activity and therefore it was not suitable as a drug.
Thus the goal was to develop a smaller nonpeptide substance with similar inhibition and binding features. At this time, a group at DuPont had already started the screening of nonpeptide mimics of Ang II using existing substances from chemical libraries.
Research investigators at Takeda
is a Japanese family name.1990 Census Name Files< ...
discovered in 1982 the weak nonpeptide Ang II antagonists S-8307 and S-8308 from a group of 1- benzylimidazole-5-acetic acid derivatives. S-8307 and S-8308 have moderate potency
Potency may refer to:
* Potency (pharmacology), a measure of the activity of a drug in a biological system
* Virility
* Cell potency, a measure of the differentiation potential of stem cells
* In homeopathic dilutions, potency is a measure of how ...
, short duration of action and limited oral bioavailability, however they are selective and competitive AT1 receptor antagonists without partial agonist activity. A group at DuPont postulated that both Ang II and the Takeda leads were bound at the same receptor site. These two substances served as lead compounds for further optimization of AT1 receptor blockers.
Using nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
studies on the spatial structure of Ang II, scientists at DuPont discovered that the Takeda structures had to be enlarged at a particular position to resemble more closely the much larger peptide Ang II.
Computer modeling was used to compare S-8308 and S-8307 with Ang II and it was seen that Ang II contains two acidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
residues near the NH2 terminus. These groups were not mimicked by the Takeda leads and therefore it was hypothesized that acidic functional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
would have to be added to the compounds.The 4-carboxy-derivative EXP-6155 had a binding activity which was ten-fold greater than that of S-8308 which further strengthened this
hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
.
By replacing the 4-carboxy-group with a 2-carboxy-benzamido-moiety the compound EXP-6803 was synthesized. It had highly increased binding affinity but was only active when administered intravenously
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrie ...
.
Replacing the 2-carboxy-benzamido-group with a 2-carboxy-phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
-group created the lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipo ...
biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
-containing EXP-7711, which exhibited good oral activity but slightly less affinity for the AT1 receptor. Then the polar carboxyl group was replaced with a more lipophilic
tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be fo ...
group in order to increase oral bioavailability and duration of action further and the compound thus formed was named losartan. This development took place in 1986 and losartan became the first successful Ang II antagonist drug, approved as such in the United States in 1995 and was marketed by Merck
Merck refers primarily to the German Merck family and three companies founded by the family, including:
* the Merck Group, a German chemical, pharmaceutical and life sciences company founded in 1668
** Merck Serono (known as EMD Serono in the Unite ...
.
This development was an extensive program and it is estimated that the process from the Takeda structures to the final substance, losartan, took more than fifty person-years of work in biological testing and chemical modifications. This represents an excellent investment given that a recent study estimated that losartan administration in the European union may reduce health care provision costs by 2.5 billion euro over 3.5 years.
Using a different lead, optimization from S-8308, eprosartan
Eprosartan is an angiotensin II receptor antagonist used for the treatment of high blood pressure. It is marketed in the United States as ''Teveten'' by Abbvie, the spin-off of the pharmaceutical discovery division of Abbott Laboratories; it i ...
was developed by SmithKline Beecham
GSK plc, formerly GlaxoSmithKline plc, is a British multinational pharmaceutical and biotechnology company with global headquarters in London, England. Established in 2000 by a merger of Glaxo Wellcome and SmithKline Beecham. GSK is the tent ...
in 1992. Eprosartan does not have a biphenyl-methyl structure but in order to mimic the C-terminal end of Ang II the 5-acetic acid group was replaced with an ''a''-thienylacrylic acid and a 4-carboxy-moiety. Eprosartan is a selective, potent and competitive AT1 antagonist and its binding to AT1 receptors is rapid, reversible, saturable and of high affinity.
Development from losartan to other drugs
Losartan,valsartan
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARB ...
, candesartan
Candesartan is an angiotensin receptor blocker used mainly for the treatment of high blood pressure and congestive heart failure. Candesartan has a very low maintenance dose. The metabolism for the drug is unique as it is a cascading prodrug. Ca ...
, irbesartan
Irbesartan, sold under the brand name Avapro among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versi ...
, telmisartan
Telmisartan, sold under the brand name Micardis among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth ...
and olmesartan
Olmesartan, sold under the trade name Benicar among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versi ...
all contain a biphenyl- methyl group.
Losartan is partly metabolized to its 5- carboxylic acid metabolite EXP 3174, which is a more potent AT1 receptor antagonist than its parent compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
and has been a model for the continuing development of several other ARBs.
Valsartan, candesartan and irbesartan were all developed in 1990.
Valsartan, first marketed by Novartis
Novartis AG is a Swiss-American multinational pharmaceutical corporation based in Basel, Switzerland and
Cambridge, Massachusetts, United States (global research).name="novartis.com">https://www.novartis.com/research-development/research-loc ...
, is a nonheterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
ARB, where the imidazole of losartan has been replaced by an acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC ...
ated amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
.
Irbesartan was developed by Sanofi
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. Originally, the corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Syn ...
Research and is longer acting than valsartan and losartan and it has an imidazolinone ring where a carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
group functions as a hydrogen bond acceptor instead of the hydroxymethyl group in losartan. Irbesartan is a non-competitive inhibitor.
Candesartan cilexetil
Candesartan is an angiotensin receptor blocker used mainly for the treatment of high blood pressure and congestive heart failure. Candesartan has a very low maintenance dose. The metabolism for the drug is unique as it is a cascading prodrug. Ca ...
(TCV 116) is a benzimidazole which was developed at Takeda and is an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
prodrug. In vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
, it is rapidly converted to the much more potent corresponding 7-carboxylic acid, candesartan. In the interaction of candesartan with AT1 receptor the carboxyl group of the benzimidazole ring plays an important role. Candesartan and its prodrug have stronger blood pressure lowering effects than EXP 3174 and losartan.
Telmisartan, which was discovered and developed in 1991 by Boehringer Ingelheim, has carboxylic acid as the biphenyl acidic group. It has the longest elimination half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of the ARBs or about 24 hours.
Olmesartan medoxomil was developed by Sankyo
is a Japanese company, and one of the three major pachinko
is a mechanical game originating in Japan that is used as an arcade game, and much more frequently for gambling. Pachinko fills a niche in Japanese gambling comparable to that ...
in 1995 and is the newest ARB on the market, marketed in 2002. It is an ester prodrug like candesartan cilexetil. In vivo, the prodrug is completely and rapidly hydrolyzed to the active acid form, olmesartan (RNH-6270). It has a hydroxyisopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often ...
group connected to the imidazole ring in addition to the carboxyl group.
Pharmacophore and structure-activity relationship
PharmacophoreThere are three functional groups that are the most important parts for the
bioactivity
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or ...
of ARBs, see figure 1 for details.The first one is the imidazole ring that binds to amino acids in helix 7 ( Asn295). The second group is the biphenyl-methyl group that binds to amino acids in both helices 6 and 7 ( Phe301, Phe300, Trp253 and
His
His or HIS may refer to:
Computing
* Hightech Information System, a Hong Kong graphics card company
* Honeywell Information Systems
* Hybrid intelligent system
* Microsoft Host Integration Server
Education
* Hangzhou International School, in ...
256). The third one is the tetrazole
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be fo ...
group that interacts with amino acids in helices 4 and 5 ( Arg167 and Lys199). The tetrazole group has been successfully replaced by a carboxylic acid group as is the case with telmisartan. Structure-activity relationship (SAR)
Most of the ARBs have the same
pharmacophore
300px, An example of a pharmacophore model.
A pharmacophore is an abstract description of molecular features that are necessary for molecular recognition of a ligand by a biological macromolecule. IUPAC defines a pharmacophore to be "an ensemble o ...
so the difference in their biochemical and physiological effects is mostly due to different substituents. Activity of a drug is dependent of its affinity for the substrate site and the length of time it binds to the site.
Lipophilic substituents like the linear alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
group at the 2-position on the imidazole ring together with the biphenyl-methyl group, associate with hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
pockets of the receptor. An acidic group like tetrazole, CO2H or NHSO2CF3 at the 1-position of the biphenyl-methyl group will bind to a basic position in the receptor and are required for potent antagonistic activity. In valsartan, the imidazole ring of losartan has been replaced with an acylated amino acid.
Several substituents have been tried at the 4- and 5- positions on the imidazole ring. The chloro and hydroxymethyl groups connected to these positions in losartan are probably not of much importance in receptor binding since the other ARBs do not possess these functional groups and have comparable or better binding affinities than losartan. Irbesartan has a carbonyl group at the 5-position, functioning as a hydrogen bond acceptor in place of the hydroxymethyl group of losartan, resulting in a longer binding to the receptor.
The structure of eprosartan is the one that differs most from the other ARBs, the usual biphenyl-methyl group has been replaced by a carboxy
benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
group that mimics more closely the phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
ic moiety of Tyr4 group of Ang II. This change results in a stronger binding to the receptor but the biochemical and physiological effects are not significantly improved. Telmisartan has a carboxylic acid at the 2-position of the biphenyl-methyl group and is more potent than the tetrazole analogue.
It has been reported that
imidazoles
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
that have hydroxymethyl and carboxy groups at the 4- and 5 position, possessed potent antagonistic activity, caused by the hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
and hydrophilicity
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
of the hydroxymethyl group.It has also been reported that an hydroxy group in the 4-position on the imidazole ring, plays an important role in the binding affinity and compensates for the disadvantage of
lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
of the bulky alkyl group.These results show that a medium-sized hydroxy alkyl group, such as CHMeOH and CMe2OH, is favorable for the substituent of the 4-position on the imidazole ring. Furthermore, the
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
izable group is favorable for the binding affinity.
Candesartan and olmesartan have the highest affinity for the AT1 receptors, followed by irbesartan and eprosartan. Valsartan, telmisartan and EXP 3174 have similar affinities that are about ten-fold less than that of candesartan. Losartan has the least affinity. ARBs' affinity for the AT2 receptor is generally much lower (or around 10,000 times less) than for the AT1 subtype. Therefore, they allow unhindered stimulation of the AT2 receptor.
Drug comparison and pharmacokinetics
ARBs have a largetherapeutic index
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes ...
and therefore their (mostly low) oral bioavailability does not appear to be of clinical significance.
As can be seen in table 1, these drugs are highly plasma protein-bound and therefore oral administration once a day should provide sufficient antihypertensive
Antihypertensives are a class of drugs that are used to treat hypertension (high blood pressure). Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke and myocardial infarction. Evidence suggests tha ...
effects.
Around 14% of orally ingested losartan is metabolized to its 5-carboxylic acid metabolite EXP 3174. As mentioned before, candesartan cilexetil and olmesartan medoxomil are inactive ester prodrugs that are completely hydrolyzed to their active forms by esterases
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
A wide range of different esterases exist that differ in their substrate specificity, their protein structur ...
during absorption from the gastrointestinal tract. These three metabolites are more potent AT1 receptor antagonists than their prodrugs
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug ...
. The other ARBs do not have active metabolites.
All of the ARBs, except for valsartan and olmesartan, are metabolized in some way by the cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various co ...
(CYP) enzyme 2C9, that is found in the human liver. CYP2C9
Cytochrome P450 family 2 subfamily C member 9 (abbreviated CYP2C9) is an enzyme protein. The enzyme is involved in metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the prote ...
is for example responsible for the metabolizing of losartan to EXP 3174 and the slow metabolizing of valsartan and candesartan to their inactive metabolites. Telmisartan is, on the other hand, in part metabolized by glucuronidation
Glucuronidation is often involved in drug metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve gl ...
and olmesartan is excreted as the unchanged drug.
Telmisartan is the only ARB that can cross the blood–brain barrier and can therefore inhibit centrally mediated effects of Ang II, contributing to even better blood pressure control.
All of the ARBs have the same mechanism of action
In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targ ...
and differences in their potency can be related to their different pharmacokinetic profiles. A few clinical head-to-head comparisons have been made and candesartan, irbesartan and telmisartan appear to be slightly more effective than losartan in lowering blood pressure. This difference may be related to different strengths of activity at the receptor level, such as duration and strength of receptor binding.
ARBs under development
Other attributes of ARBs are also under investigation, such as the positive effects of telmisartan on
lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids includ ...
and glucose metabolism
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.
Carbohydrates are central to many essential metabolic pathways. Pla ...
and losartan's effects of lowering uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown ...
levels. Such effects might lead to new indications for these drugs but further research is needed.
See also
* Discovery and development of renin inhibitorsReferences
{{Angiotensin receptor modulators Angiotensin II receptor antagonists Angiotensin Receptor Blockers, Discovery And Development Of