|
Esterases
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis. A wide range of different esterases exist that differ in their substrate specificity, their protein structure, and their biological function. EC classification/list of enzymes * ''EC 3.1.1'': Carboxylic ester hydrolases ** Acetylesterase (EC 3.1.1.6), splits off acetyl groups *** Cholinesterase **** Acetylcholinesterase, inactivates the neurotransmitter acetylcholine **** Pseudocholinesterase, broad substrate specificity, found in the blood plasma and in the liver ** Pectinesterase (EC 3.1.1.11), clarifies fruit juices * ''EC 3.1.2'': Thiolester hydrolases ** Thioesterase *** Ubiquitin carboxy-terminal hydrolase L1 * ''EC 3.1.3'': Phosphoric monoester hydrolases ** Phosphatase (EC 3.1.3.x), hydrolyses phosphoric acid monoesters into a phosphate ion and an alcohol *** Alkaline phosphatase, removes phosphate groups from many types of mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pectinesterase
Pectinesterase (EC 3.1.1.11; systematic name pectin pectylhydrolase) is a ubiquitous cell-wall-associated enzyme that presents several isoforms that facilitate plant cell wall modification and subsequent breakdown. It catalyzes the following reaction: :pectin + ''n'' H2O = ''n'' methanol + pectate It is found in all higher plants as well as in some bacteria and fungi. Pectinesterase functions primarily by altering the localised pH of the cell wall resulting in alterations in cell wall integrity. Pectinesterase catalyses the de-esterification of pectin into pectate and methanol. Pectin is one of the main components of the plant cell wall. In plants, pectinesterase plays an important role in cell wall metabolism during fruit ripening. In plant bacterial pathogens such as '' Erwinia carotovora'' and in fungal pathogens such as '' Aspergillus niger'', pectinesterase is involved in maceration and soft-rotting of plant tissue. Plant pectinesterases are regulated by pectinesterase i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphodiesterase
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, ''phosphodiesterase'' refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many other families of phosphodiesterases, including phospholipases C and D, autotaxin, sphingomyelin phosphodiesterase, DNases, RNases, and restriction endonucleases (which all break the phosphodiester backbone of DNA or RNA), as well as numerous less-well-characterized small-molecule phosphodiesterases. The cyclic nucleotide phosphodiesterases comprise a group of enzymes that degrade the phosphodiester bond in the second messenger molecules cAMP and cGMP. They regulate the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. PDEs are therefore important regulators of signal transduction mediated by these second messenger molecules. History These multiple forms (isoforms or subtypes) of ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioesterase
Thioesterases are enzymes which belong to the esterase family. Esterases, in turn, are one type of the several hydrolases known. Thioesterases exhibit esterase activity (splitting of an ester into acid and alcohol, in the presence of water) specifically at a thiol group. Thioesterases or thiolester hydrolases are identified as members of EC 3.1.2. Family The thioesterase activity is performed by members of the acyl-CoA thioesterase (ACOT) family. The regulatory role of ACOT in fatty acid metabolism depends on their substrate specificity, tissue expression and subcellular localization. For example, deactivation of fatty acids at the ER may traffic fatty acids away from pathways associated with the ER membrane, such as glycerolipid biosynthesis. Two structurally different ACOT types lead to a similar enzymatic activity in vitro, dividing the family into type I and type II ACOTs. Type I ACOTs (ACOT1–6) contain the α/β-hydrolase domain, which is also present in many lipases a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
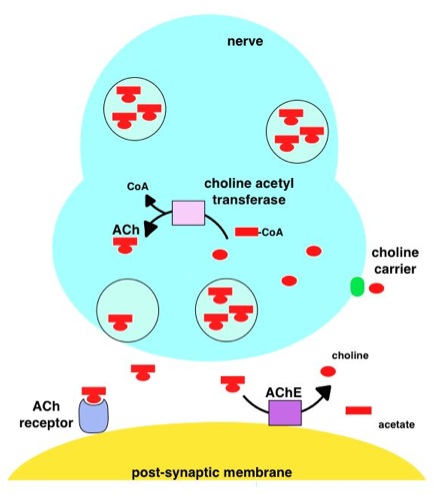
Acetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters: : acetylcholine + H2O = choline + acetate It is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to the carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. Enzyme structure and mechanism AChE is a hydrolase that hydrolyzes choline esters. It has a very high catalytic activity—each molecule of AChE degrades about 25,000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. The active site of AChE comprises 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyrylcholinesterase
Butyrylcholinesterase ( HGNC symbol BCHE; EC 3.1.1.8), also known as BChE, BuChE, BuChase, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the ''BCHE'' gene. It is very similar to the neuronal acetylcholinesterase, which is also known as RBC or erythrocyte cholinesterase. The term "serum cholinesterase" is generally used in reference to a clinical test that reflects levels of both of these enzymes in the blood. Assay of butyrylcholinesterase activity in plasma can be used as a liver function test as both hypercholinesterasemia and hypocholinesterasemia indicate pathological processes. The half-life of BCHE is approximately 10 to 14 days. Butyrylcholine is a synthetic compound that does not occur in the body naturally. It is used as a tool to distinguish between acetylcholinesterase and butyrylcholinesteras ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Monoester Hydrolases
Phosphoric monoester hydrolases (or phosphomonoesterases) are enzymes that catalyse the hydrolysis of O-P bonds by nucleophilic attack of phosphorus by cysteine residues or coordinated metal ions. They are categorized with the EC number 3.1.3. Examples include: * acid phosphatase * alkaline phosphatase * fructose-bisphosphatase * glucose-6-phosphatase * phosphofructokinase-2 * phosphoprotein phosphatase * calcineurin * 6-phytase See also * phosphodiesterase * phosphatase In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate ... External links * Metabolism {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Phosphatase
The enzyme alkaline phosphatase (EC 3.1.3.1, alkaline phosphomonoesterase; phosphomonoesterase; glycerophosphatase; alkaline phosphohydrolase; alkaline phenyl phosphatase; orthophosphoric-monoester phosphohydrolase (alkaline optimum), systematic name phosphate-monoester phosphohydrolase (alkaline optimum)) catalyses the following reaction: : a phosphate monoester + H2O = an alcohol + phosphate Alkaline phosphatase has the physiological role of dephosphorylating compounds. The enzyme is found across a multitude of organisms, prokaryotes and eukaryotes alike, with the same general function but in different structural forms suitable to the environment they function in. Alkaline phosphatase is found in the periplasmic space of '' E. coli'' bacteria. This enzyme is heat stable and has its maximum activity at high pH. In humans, it is found in many forms depending on its origin within the body – it plays an integral role in metabolism within the liver and development withi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid Monoesters
Phosphomonoesters (or phosphoric esters) are chemical compounds containing one ester bond and a phosphate group. In biology, phosphomonoesters are needed as the building blocks for the synthesis of Phospholipid cellular membranes, especially those found on neurons. Enzymes which cleave these bonds are known as phosphomonoesterases, or phosphatases. See also * Phosphoric acid * Phospholipid Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ... References External links CRISP Organophosphates {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholinesterase
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters: : an acylcholine + H2O = choline + a carboxylate Several of these serve as neurotransmitters. Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid. These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase (also called choline esterase I or erythrocyte cholinesterase); it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholinester ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitin Carboxy-terminal Hydrolase L1
Ubiquitin carboxy-terminal hydrolase L1 (, ''ubiquitin C-terminal hydrolase'', ''UCH-L1'') is a deubiquitinating enzyme. Function UCH-L1 is a member of a gene family whose products hydrolyze small C-terminal adducts of ubiquitin to generate the ubiquitin monomer. Expression of UCH-L1 is highly specific to neurons and to cells of the diffuse neuroendocrine system and their tumors. It is abundantly present in all neurons (accounts for 1-2% of total brain protein), expressed specifically in neurons and testis/ovary. The catalytic triad of UCH-L1 contains a cysteine at position 90, an aspartate at position 176, and a histidine at position 161 that are responsible for its hydrolase activity. Relevance to neurodegenerative disorders A point mutation (I93M) in the gene encoding this protein is implicated as the cause of Parkinson's disease in one German family, although this finding is controversial, as no other Parkinson's disease patients with this mutation have been found. Fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylesterase
The enzyme acetylesterase () catalyzes the reaction :an acetic ester + H2O \rightleftharpoons an alcohol + acetate This enzyme belongs to the family of hydrolases, specifically those acting on carboxylic ester bonds. The systematic name of this enzyme class is acetic-ester acetylhydrolase. Other names in common use include C-esterase (in animal tissues), acetic ester hydrolase, chloroesterase, ''p''-nitrophenyl acetate esterase, and Citrus acetylesterase. Structural studies As of late 2007, 3 structures A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ... have been solved for this class of enzymes, with PDB accession codes , , and . References * * * EC 3.1.1 Enzymes of known structure {{3.1-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolase
Hydrolase is a class of enzyme that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases. Esterases cleave ester bonds in lipids and phosphatases cleave phosphate groups off molecules. An example of crucial esterase is acetylcholine esterase, which assists in transforming the neuron impulse into the acetate group after the hydrolase breaks the acetylcholine into choline and acetic acid. Acetic acid is an important metabolite in the body and a critical intermediate for other reactions such as glycolysis. Lipases hydrolyze glycerides. Glycosidases cleave sugar molecules off carbohydrates and peptidases hydrolyze peptide bonds. Nucleosidases hydrolyze the bonds of nucleotides. Hydrolase enzymes are important for the body because they h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |