|
Pseudocapacitance
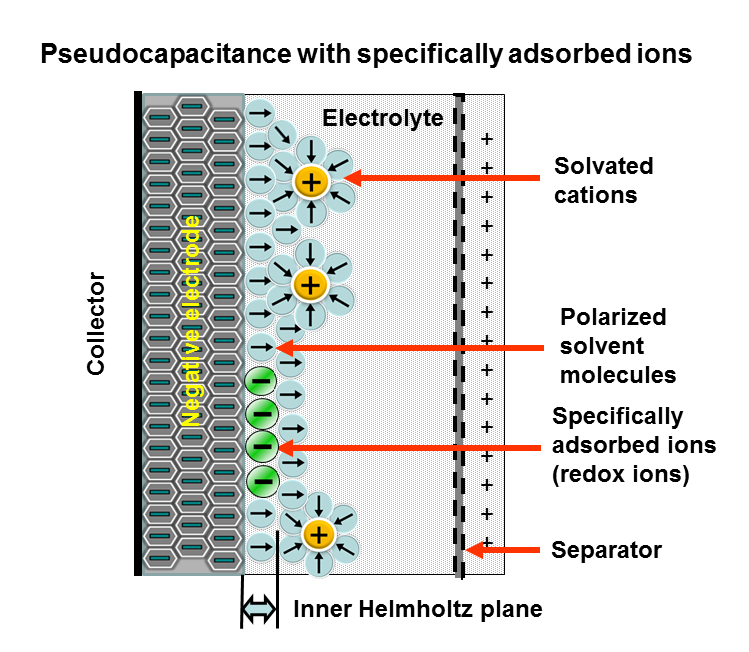
Pseudocapacitance is the Electrochemistry, electrochemical storage of electricity in an Supercapacitor, electrochemical capacitor (Pseudocapacitor). This faradaic charge transfer originates by a very fast sequence of reversible Faradaic current, faradaic redox, Capacitive deionization, electrosorption or Intercalation (chemistry), intercalation processes on the surface of suitable electrodes. see alsBrian E. Conway in Electrochemistry Encyclopedia: ''ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications''E. Frackowiak, F. Beguin: ''Carbon Materials For The Electrochemical Storage Of Energy In Capacitors.'' In: ''CARBON.'' 39, 2001, S. 937–950PDF E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: ''Nanotubular Materials For Supercapacitors.'' In: ''Journal of Power Sources.'' Volumes 97–98, Juli 2001, S. 822–825, . Pseudocapacitance is accompanied by an electron Charge transfer complex, charge-transfer between electrolyte and electrode coming from a Solvation, de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercapacitor
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Supercapacitors are used in applications requiring many rapid charge/discharge cycles, rather than long-term compact energy storage — in automobiles, buses, trains, cranes and elevators, where they are used for regenerative braking, short-term energy storage, or burst-mode power delivery. Smaller units are used as power backup for static random-access memory (SRAM). Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectric, but rather, they use electrost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercapacitors
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Supercapacitors are used in applications requiring many rapid charge/discharge cycles, rather than long-term compact energy storage — in automobiles, buses, trains, cranes and elevators, where they are used for regenerative braking, short-term energy storage, or burst-mode power delivery. Smaller units are used as power backup for static random-access memory (SRAM). Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectric, but rather, they use electrost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudocapacitor
Pseudocapacitors store electrical energy faradaically by electron charge transfer between electrode and electrolyte. This is accomplished through electrosorption, reduction-oxidation reactions ( redox reactions), and intercalation processes, termed '' pseudocapacitance''. A pseudocapacitor is part of an electrochemical capacitor, and forms together with an electric double-layer capacitor (EDLC) to create a supercapacitor. Pseudocapacitance and double-layer capacitance add up to a common inseparable capacitance value of a supercapacitor. However, they can be effective with very different parts of the total capacitance value depending on the design of the electrodes. A pseudocapacitance may be higher by a factor of 100 as a double-layer capacitance with the same electrode surface. A pseudocapacitor has a chemical reaction at the electrode, unlike EDLCs where the electrical charge storage is stored electrostatically with no interaction between the electrode and the ions. Ps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Layer (interfacial)
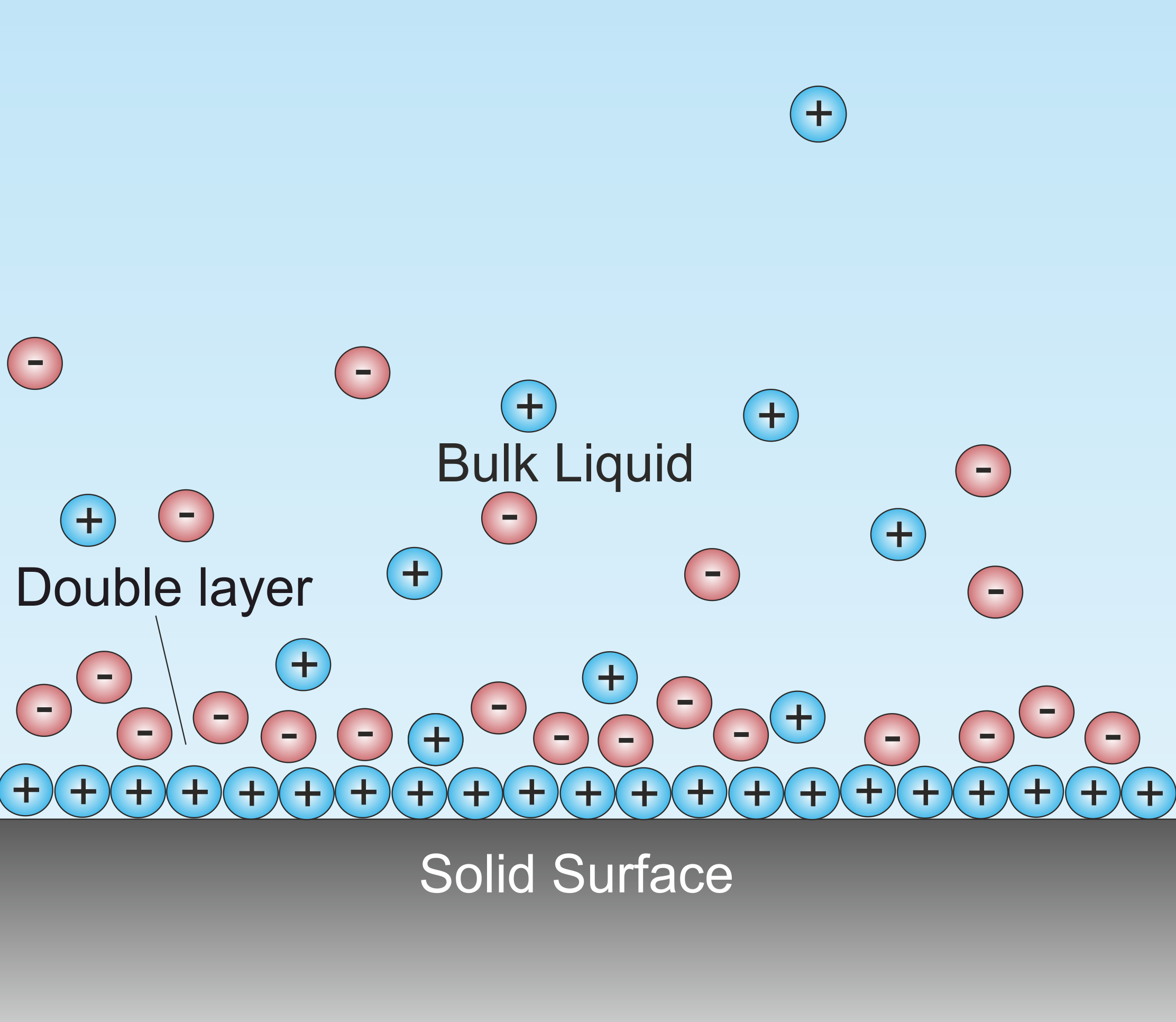
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double-layer Capacitance
Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with opposing polarity form, one at the surface of the electrode, and one in the electrolyte. These two layers, electrons on the electrode and ions in the electrolyte, are typically separated by a single layer of solvent molecules that adhere to the surface of the electrode and act like a dielectric in a conventional capacitor. The amount of electric charge stored in double-layer capacitor depends on the applied voltage. The unit of capacitance is the farad. The double-layer capacitance is the physical principle behind the electrostatic double-layer type of Supercapacitors. History * Development of the double layer and pseudocapacitance model see Double layer (interfacial) * Development of the electrochemical components see Supercapacitors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rechargeable Battery
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer (Li-ion polymer). Rechargeable batteries typically initially cost more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a test charge between the two points. In the International System of Units, the derived unit for voltage is named '' volt''. The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in generator, inductors, and transformers). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. A voltmeter can be used to measure the voltage between two points in a system. Often a common reference potential such as the ground of the system is used as one of the points. A voltage can represent either a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farad
The farad (symbol: F) is the unit of electrical capacitance, the ability of a body to store an electrical charge, in the International System of Units (SI). It is named after the English physicist Michael Faraday (1791–1867). In SI base units 1 F = 1 kg−1⋅ m−2⋅ s4⋅ A2. Definition The capacitance of a capacitor is one farad when one coulomb of charge changes the potential between the plates by one volt. Equally, one farad can be described as the capacitance which stores a one-coulomb charge across a potential difference of one volt. The relationship between capacitance, charge, and potential difference is linear. For example, if the potential difference across a capacitor is halved, the quantity of charge stored by that capacitor will also be halved. For most applications, the farad is an impractically large unit of capacitance. Most electrical and electronic applications are covered by the following SI prefixes: *1 mF (millifarad, one thousandth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Power Density
Power density is the amount of power (time rate of energy transfer) per unit volume. In energy transformers including batteries, fuel cells, motors, power supply units etc., power density refers to a volume, where it is often called volume power density, expressed as W/m3. In reciprocating internal combustion engines, power density (power per swept volume or brake horsepower per cubic centimeter) is an important metric, based on the ''internal'' capacity of the engine, not its external size. Examples See also *Surface power density, energy per unit of area * Energy density, energy per unit volume * Specific energy, energy per unit mass * Power-to-weight ratio/specific power, power per unit mass **Specific absorption rate Specific absorption rate (SAR) is a measure of the rate at which energy is absorbed per unit mass by a human body when exposed to a radio frequency (RF) electromagnetic field. It can also refer to absorption of other forms of energy by tissue, inc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Process
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some sort. In an "engineering" sense, a chemical process is a method intended to be used in manufacturing or on an industrial scale (see Industrial process) to change the composition of chemical(s) or material(s), usually using technology similar or related to that used in chemical plants or the chemical industry. Neither of these definitions are exact in the sense that one can always tell definitively what is a chemical process and what is not; they are practical definitions. There is also significant overlap in these two definition variations. Because of the inexactness of the definition, chemists and other scientists use the term "chemical process" only in a general sense or in the engineering sense. However, in the "process (engineer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have id ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |