|
Supercapacitor
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Supercapacitors are used in applications requiring many rapid charge/discharge cycles, rather than long-term compact energy storage — in automobiles, buses, trains, cranes and elevators, where they are used for regenerative braking, short-term energy storage, or burst-mode power delivery. Smaller units are used as power backup for static random-access memory (SRAM). Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectric, but rather, they use electrosta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercapacitor Types Overview
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and Rechargeable battery, rechargeable batteries. It typically stores 10 to 100 times more specific energy, energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than Rechargeable battery, rechargeable batteries. Supercapacitors are used in applications requiring many rapid charge/discharge cycles, rather than long-term compact energy storage — in automobiles, buses, trains, cranes and elevators, where they are used for Regenerative brake, regenerative braking, short-term energy storage, or burst-mode power delivery. Smaller units are used as power backup for static random-access memory (SRAM). Unlike ordinary capacitors, supercapacito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium-ion Capacitor
A lithium-ion capacitor (LIC) is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors. History In 1981, Dr. Yamabe of Kyoto University, in collaboration with Dr. Yata of Kanebo Co., created a material known as PAS (polyacenic semiconductive) by pyrolyzing phenolic resin at 400–700 °C. This amorphous carbonaceous material performs well as the electrode in high-energy-density rechargeable devices. Patents were filed in the early 1980s by Kanebo Co., and efforts to commercialize PAS capacitors and lithium-ion capacitors (LICs) began. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capacitor
A capacitor is a device that stores electrical energy in an electric field by virtue of accumulating electric charges on two close surfaces insulated from each other. It is a passive electronic component with two terminals. The effect of a capacitor is known as capacitance. While some capacitance exists between any two electrical conductors in proximity in a circuit, a capacitor is a component designed to add capacitance to a circuit. The capacitor was originally known as the condenser, a term still encountered in a few compound names, such as the ''condenser microphone''. The physical form and construction of practical capacitors vary widely and many types of capacitor are in common use. Most capacitors contain at least two electrical conductors often in the form of metallic plates or surfaces separated by a dielectric medium. A conductor may be a foil, thin film, sintered bead of metal, or an electrolyte. The nonconducting dielectric acts to increase the capacitor's c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
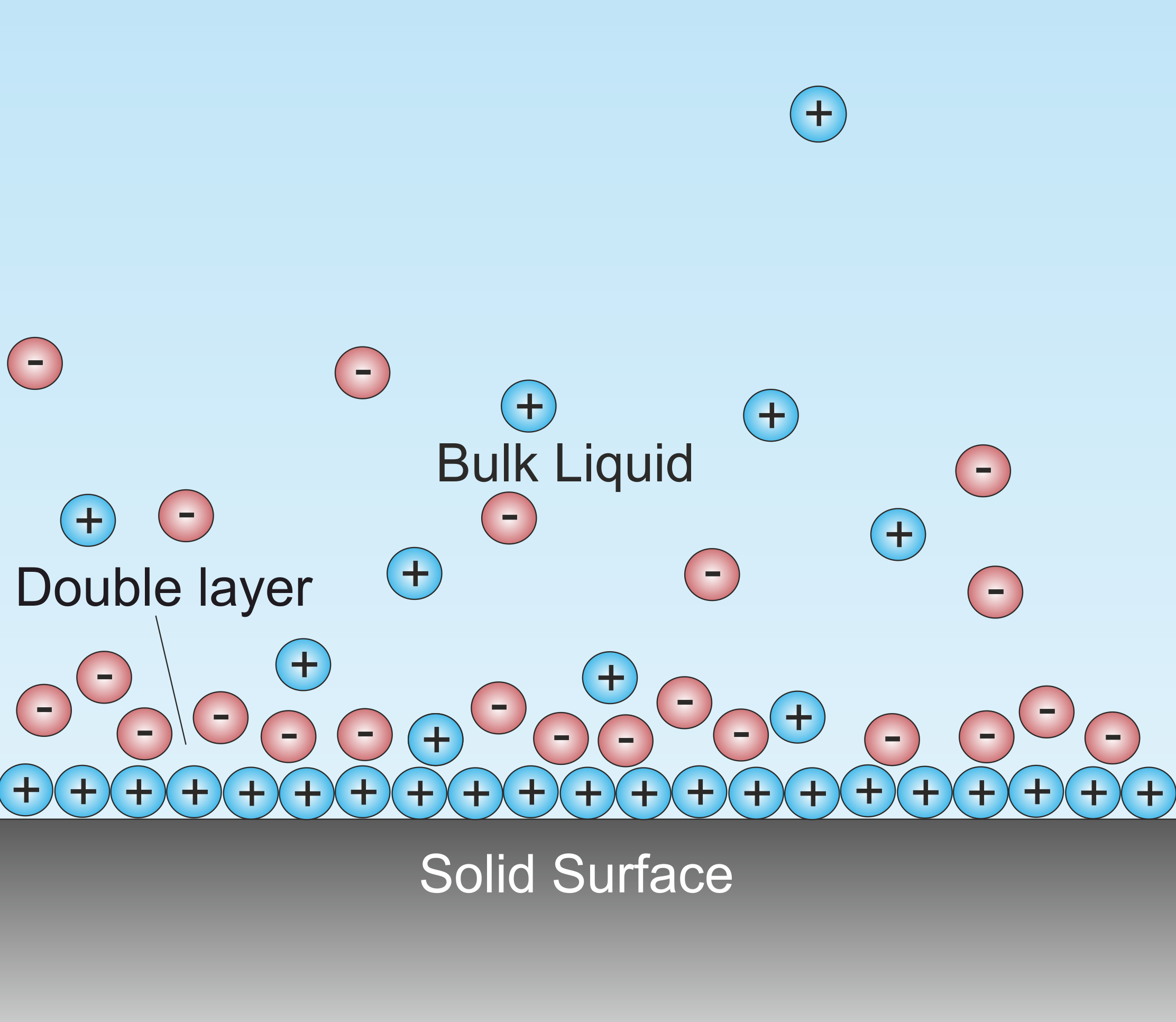
Double Layer (interfacial)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudocapacitor
Pseudocapacitors store electrical energy faradaically by electron charge transfer between electrode and electrolyte. This is accomplished through electrosorption, reduction-oxidation reactions (redox reactions), and intercalation processes, termed ''pseudocapacitance''. A pseudocapacitor is part of an electrochemical capacitor, and forms together with an electric double-layer capacitor (EDLC) to create a supercapacitor. Pseudocapacitance and double-layer capacitance add up to a common inseparable capacitance value of a supercapacitor. However, they can be effective with very different parts of the total capacitance value depending on the design of the electrodes. A pseudocapacitance may be higher by a factor of 100 as a double-layer capacitance with the same electrode surface. A pseudocapacitor has a chemical reaction at the electrode, unlike EDLCs where the electrical charge storage is stored electrostatically with no interaction between the electrode and the ions. Pseudoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regenerative Brake
Regenerative braking is an energy recovery mechanism that slows down a moving vehicle or object by converting its kinetic energy into a form that can be either used immediately or stored until needed. In this mechanism, the electric traction motor uses the vehicle's momentum to recover energy that would otherwise be lost to the brake discs as heat. This method contrasts with conventional braking systems. In those systems, the excess kinetic energy is converted to unwanted and wasted heat due to friction in the brakes, or with rheostatic brakes, where the energy is recovered by using electric motors as generators but is immediately dissipated as heat in resistors. In addition to improving the overall efficiency of the vehicle, regeneration can significantly extend the life of the braking system as the mechanical parts will not wear out quickly. General principle The most common form of regenerative brake involves an electric motor functioning as an electric generator. In elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conducting Polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that Electrical conductance, conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by Dispersion (chemistry), dispersion. Conductive polymers are generally not thermoplastics, ''i.e.'', they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques. History Polyaniline was first described in the mid-19th century by Henry Letheby, who investigated the electrochemical and chemical oxidation products of aniline in acidic media. He noted that reduced form was colourless but the oxidized forms were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytic Capacitor
An electrolytic capacitor is a polarized capacitor whose anode or positive plate is made of a metal that forms an insulating oxide layer through anodization. This oxide layer acts as the dielectric of the capacitor. A solid, liquid, or gel electrolyte covers the surface of this oxide layer, serving as the cathode or negative plate of the capacitor. Due to their very thin dielectric oxide layer and enlarged anode surface, electrolytic capacitors have a much higher capacitance-voltage (CV) product per unit volume than ceramic capacitors or film capacitors, and so can have large capacitance values. There are three families of electrolytic capacitor: aluminum electrolytic capacitors, tantalum electrolytic capacitors, and niobium electrolytic capacitors. The large capacitance of electrolytic capacitors makes them particularly suitable for passing or bypassing low-frequency signals, and for storing large amounts of energy. They are widely used for decoupling or noise filtering ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capacitance
Capacitance is the capability of a material object or device to store electric charge. It is measured by the change in charge in response to a difference in electric potential, expressed as the ratio of those quantities. Commonly recognized are two closely related notions of capacitance: ''self capacitance'' and ''mutual capacitance''. An object that can be electrically charged exhibits self capacitance, for which the electric potential is measured between the object and ground. Mutual capacitance is measured between two components, and is particularly important in the operations of the capacitor, a device designed for this purpose as an elementary Linear circuit, linear electronic component. Capacitance is a function only of the geometry of the design of the capacitor, e.g., the opposing surface area of the plates and the distance between them, and the permittivity of the dielectric material between the plates. For many dielectric materials, the permittivity and thus the capaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intercalation (chemistry)
In chemistry, intercalation is the reversible inclusion or insertion of a molecule (or ion) into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides. : Examples Graphite One famous intercalation host is graphite, which intercalates potassium as a guest. Intercalation expands the van der Waals gap between sheets, which requires energy. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e., redox. Two potassium graphite compounds are KC8 and KC24. Carbon fluorides (e.g., (CF)x and (C4F)) are prepared by reaction of fluorine with graphitic carbon. The color is greyish, white, or yellow. The bond between the carbon and fluorine atoms is covalent, thus fluorine is not intercalated. Such materials have been considered as a cathode in various lithium batteries. Treating graphite with strong acids in the presence of oxidizing agents causes the graphite to oxidise. Graphite bisulfate, 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)