Pseudocapacitance on:
[Wikipedia]
[Google]
[Amazon]
 Pseudocapacitance is the
Pseudocapacitance is the
Brian E. Conway in Electrochemistry Encyclopedia: ''ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications''
E. Frackowiak, F. Beguin: ''Carbon Materials For The Electrochemical Storage Of Energy In Capacitors.'' In: ''CARBON.'' 39, 2001, S. 937–950
PDF
E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: ''Nanotubular Materials For Supercapacitors.'' In: ''Journal of Power Sources.'' Volumes 97–98, Juli 2001, S. 822–825, . Pseudocapacitance is accompanied by an
 A fundamental difference between redox reactions in batteries and in electrochemical capacitors (supercapacitors) is that in the latter, the reactions are a very fast sequence of reversible processes with electron transfer without any phase changes of the electrode molecules. They do not involve making or breaking
A fundamental difference between redox reactions in batteries and in electrochemical capacitors (supercapacitors) is that in the latter, the reactions are a very fast sequence of reversible processes with electron transfer without any phase changes of the electrode molecules. They do not involve making or breaking

 Applying a voltage at the capacitor terminals moves the polarized
Applying a voltage at the capacitor terminals moves the polarized
Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid components
/ref>B. E. Conway, V. Birss, J. Wojtowicz
The role and the utilization of pseudocapacitance for energy storage by supercapacitors
Journal of Power Sources, Volume 66, Issues 1–2, May–June 1997, Pages 1–14 When discharging pseudocapacitance, the charge transfer is reversed and the ions or atoms leave the double-layer and spread throughout the electrolyte.
Materials for electrochemical capacitors, nature materials
VOL 7, NOVEMBER 2008 During charge and discharge, ( protons) are incorporated into or removed from the
Carbon properties and their role in supercapacitors
, Journal of Power Sources 157 (2006) 11–27B.P. Bakhmatyuk, B.Ya. Venhryn, I.I. Grygorchak, M.M. Micov and S.I. Mudry
INTERCALATION PSEUDO-CAPACITANCE IN CARBON SYSTEMS OF ENERGY STORAGE
/ref>P. Simon, A. Burke
Nanostructured carbons: Double-Layer capacitance and more
 Pseudocapacitance properties can be expressed in a cyclic voltammogram. For an ideal double-layer capacitor, the current flow is reversed immediately upon reversing the potential yielding a rectangular-shaped voltammogram, with a current independent of the electrode potential. For double-layer capacitors with resistive losses, the shape changes to a
Pseudocapacitance properties can be expressed in a cyclic voltammogram. For an ideal double-layer capacitor, the current flow is reversed immediately upon reversing the potential yielding a rectangular-shaped voltammogram, with a current independent of the electrode potential. For double-layer capacitors with resistive losses, the shape changes to a
Carbon materials for the electrochemical storage of energy in Capacitors
/ref>Why does an ideal capacitor give rise to a rectangular cyclic voltammogram
/ref>
 Pseudocapacitance is the
Pseudocapacitance is the electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
storage of electricity in an electrochemical capacitor
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable ba ...
(Pseudocapacitor
Pseudocapacitors store electrical energy faradaically by electron charge transfer between electrode and electrolyte. This is accomplished through electrosorption, reduction-oxidation reactions (redox reactions), and intercalation processes, ...
). This faradaic charge transfer originates by a very fast sequence of reversible faradaic redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
, electrosorption or intercalation
Intercalation may refer to:
* Intercalation (chemistry), insertion of a molecule (or ion) into layered solids such as graphite
*Intercalation (timekeeping), insertion of a leap day, week or month into some calendar years to make the calendar foll ...
processes on the surface of suitable electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s. see alsBrian E. Conway in Electrochemistry Encyclopedia: ''ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications''
E. Frackowiak, F. Beguin: ''Carbon Materials For The Electrochemical Storage Of Energy In Capacitors.'' In: ''CARBON.'' 39, 2001, S. 937–950
E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: ''Nanotubular Materials For Supercapacitors.'' In: ''Journal of Power Sources.'' Volumes 97–98, Juli 2001, S. 822–825, . Pseudocapacitance is accompanied by an
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
. One electron per charge unit is involved. The adsorbed ion has no chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
with the atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
s of the electrode (no chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s arise) since only a charge-transfer takes place.
Faradaic pseudocapacitance only occurs together with static double-layer capacitance Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with oppos ...
. Pseudocapacitance and double-layer capacitance both contribute inseparably to the total capacitance value.
The amount of pseudocapacitance depends on the surface area, material and structure of the electrodes. Pseudocapacitance may contribute more capacitance than double-layer capacitance for the same surface area by 100x.
The amount of electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respecti ...
stored in a pseudocapacitance is linearly proportional to the applied voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge t ...
. The unit of pseudocapacitance is farad
The farad (symbol: F) is the unit of electrical capacitance, the ability of a body to store an electrical charge, in the International System of Units (SI). It is named after the English physicist Michael Faraday (1791–1867). In SI base unit ...
.
History
* Development of the double layer and pseudocapacitance model seeDouble layer (interfacial)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The ...
* Development of the electrochemical components see Supercapacitors
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeabl ...
Redox reactions
Differences
Rechargeable batteries
Redox reactions inbatteries
Battery most often refers to:
* Electric battery, a device that provides electrical power
* Battery (crime), a crime involving unlawful physical contact
Battery may also refer to:
Energy source
*Automotive battery, a device to provide power t ...
with faradaic charge-transfer between an electrolyte and the surface of an electrode were characterized decades ago. These chemical process
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some ...
es are associated with chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
s of the electrode materials usually with attendant phase changes
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states ...
. Although these chemical processes are relatively reversible, battery charge/discharge cycles often irreversibly produce unreversed chemical reaction products of the reagents. Accordingly, the cycle-life of rechargeable batteries is usually limited. Further, the reaction products lower power density
Power density is the amount of power (time rate of energy transfer) per unit volume.
In energy transformers including batteries, fuel cells, motors, power supply units etc., power density refers to a volume, where it is often called volume ...
. Additionally, the chemical processes are relatively slow, extending charge/discharge times.
Electro-chemical capacitors
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s. The de-solvated atoms or ions contributing the pseudocapacitance simply cling to the atomic structure of the electrode and charges are distributed on surfaces by physical adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
processes. Compared with batteries, supercapacitor faradaic processes are much faster and more stable over time, because they leave only traces of reaction products. Despite the reduced amount of these products, they cause capacitance degradation. This behavior is the essence of pseudocapacitance.
Pseudocapacitive processes lead to a charge-dependent, linear capacitive behavior, as well as the accomplishment of non-faradaic double-layer capacitance in contrast to batteries, which have a nearly charge-independent behavior. The amount of pseudocapacitance depends on the surface area, material and structure of the electrodes. The pseudocapacitance may exceed the value of double-layer capacitance for the same surface area by 100x.
Capacitance functionality

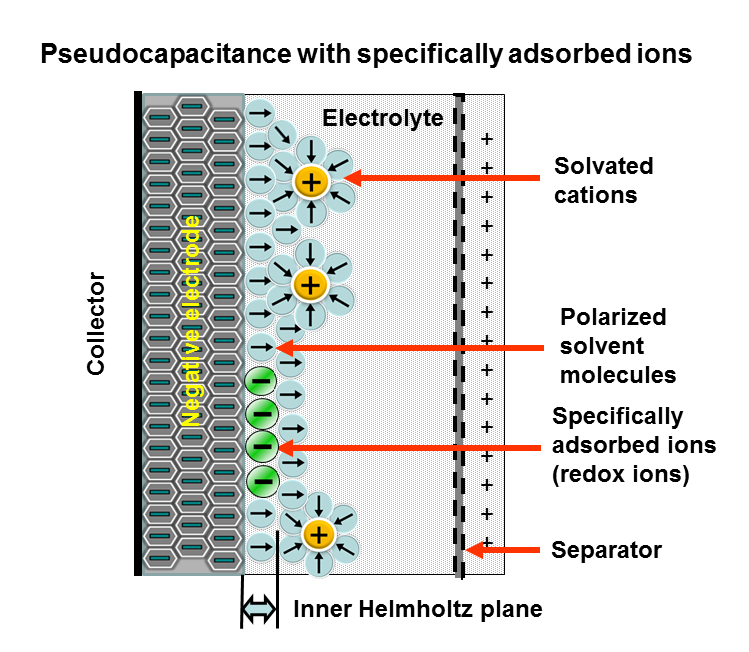
 Applying a voltage at the capacitor terminals moves the polarized
Applying a voltage at the capacitor terminals moves the polarized ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s or charged atoms in the electrolyte to the opposite polarized electrode. Between the surfaces of the electrodes and the adjacent electrolyte an electric double-layer forms. One layer of ions on the electrode surface and the second layer of adjacent polarized and solvated ions in the electrolyte move to the opposite polarized electrode. The two ion layers are separated by a single layer of electrolyte molecules. Between the two layers, a static electric field forms that results in double-layer capacitance Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with oppos ...
. Accompanied by the electric double-layer, some de-solvated electrolyte ions pervade the separating solvent layer and are adsorbed
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
by the electrode's surface atoms. They are specifically adsorbed and deliver their charge to the electrode. In other words, the ions in the electrolyte within the Helmholtz double-layer also act as electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent che ...
s and transfer electrons to the electrode atoms, resulting in a faradaic current. This faradaic charge transfer, originated by a fast sequence of reversible redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reactions, electrosorptions or intercalation
Intercalation may refer to:
* Intercalation (chemistry), insertion of a molecule (or ion) into layered solids such as graphite
*Intercalation (timekeeping), insertion of a leap day, week or month into some calendar years to make the calendar foll ...
processes between electrolyte and the electrode surface is called pseudocapacitance.
Depending on the electrode's structure or surface material, pseudocapacitance can originate when specifically adsorbed ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s pervade the double-layer, proceeding in several one-electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
stages. The electrons involved in the faradaic processes are transferred to or from the electrode's valence-electron states ( orbitals) and flow through the external circuit to the opposite electrode where a second double-layer with an equal number of opposite-charged ions forms. The electrons remain in the strongly ionized and electrode surface's "electron hungry" transition-metal ions and are not transferred to the adsorbed ions. This kind of pseudocapacitance has a linear function within narrow limits and is determined by the potential-dependent degree of surface coverage of the adsorbed anions. The storage capacity of the pseudocapacitance is limited by the finite quantity of reagent or of available surface.
Systems that give rise to pseudocapacitance:
* Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
system: Ox + ze‾ ⇌ Red
* Intercalation
Intercalation may refer to:
* Intercalation (chemistry), insertion of a molecule (or ion) into layered solids such as graphite
*Intercalation (timekeeping), insertion of a leap day, week or month into some calendar years to make the calendar foll ...
system: in ""
* Electrosorption, underpotential deposition of metal adatoms or H: + ze‾ + S ⇌ SM or + e‾ + S ⇌ SH (S = surface lattice sites)
All three types of electrochemical processes have appeared in supercapacitors.B.E. Conway, W.G. PellDouble-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid components
/ref>B. E. Conway, V. Birss, J. Wojtowicz
The role and the utilization of pseudocapacitance for energy storage by supercapacitors
Journal of Power Sources, Volume 66, Issues 1–2, May–June 1997, Pages 1–14 When discharging pseudocapacitance, the charge transfer is reversed and the ions or atoms leave the double-layer and spread throughout the electrolyte.
Materials
Electrodes' ability to produce pseudocapacitance strongly depends on the electrode materials' chemical affinity to the ions adsorbed on the electrode surface as well as on the electrode pore structure and dimension. Materials exhibiting redox behavior for use as pseudocapacitor electrodes are transition-metal oxides inserted by doping in the conductive electrode material such as active carbon, as well as conducting polymers such aspolyaniline
Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one ...
or derivatives of polythiophene
Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents ...
covering the electrode material.
Transition metal oxides/sulfides
These materials provide high pseudocapacitance and were thoroughly studied by Conway. Many oxides of transition metals likeruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemic ...
(), iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
(), iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
(), manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy u ...
() or sulfides such as titanium sulfide
Titanium(II) sulfide (TiS) is an inorganic chemical compound of titanium and sulfur.
A meteorite, Yamato 691, contains tiny flecks of this compound, making it a new mineral called wassonite
Wassonite is an extremely rare titanium sulfide mineral ...
() or their combinations generate faradaic electron–transferring reactions with low conducting resistance.
Ruthenium dioxide
Ruthenium(IV) oxide is the inorganic compound with the formula Ru O2. This black solid is the most common oxide of ruthenium. It is widely used as an electrocatalyst for producing chlorine, chlorine oxides, and O2. Like many dioxides, RuO2 adopt ...
() in combination with sulfuric acid () electrolyte provides one of the best examples of pseudocapacitance, with a charge/discharge over a window of about 1.2 V per electrode. Furthermore, the reversibility on these transition metal electrodes is excellent, with a cycle life of more than several hundred-thousand cycles. Pseudocapacitance originates from a coupled, reversible redox reaction with several oxidation steps with overlapping potential. The electrons mostly come from the electrode's valence orbital
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair for ...
s. The electron transfer reaction is very fast and can be accompanied with high currents.
The electron transfer reaction takes place according to:
: where P. Simon, Y.Gogotsi,Materials for electrochemical capacitors, nature materials
VOL 7, NOVEMBER 2008 During charge and discharge, ( protons) are incorporated into or removed from the
crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
, which generates storage of electrical energy without chemical transformation. The OH groups are deposited as a molecular layer on the electrode surface and remain in the region of the Helmholtz layer. Since the measurable voltage from the redox reaction is proportional to the charged state, the reaction behaves like a capacitor rather than a battery, whose voltage is largely independent of the state of charge.
Conducting polymers
Another type of material with a high amount of pseudocapacitance is electron-conducting polymers.Conductive polymer
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymer ...
such as polyaniline
Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one ...
, polythiophene
Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents ...
, polypyrrole
Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields.
History
...
and polyacetylene
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit . The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound ...
have a lower reversibility of the redox processes involving faradaic charge transfer than transition metal oxides, and suffer from a limited stability during cycling. Such electrodes employ electrochemical doping or dedoping of the polymers with anions and cations. Highest capacitance and power density are achieved with a n/p-type polymer configuration, with one negatively charged (n-doped) and one positively charged (p-doped) electrode.
Structure
Pseudocapacitance may originate from the electrode structure, especially from the material pore size. The use ofcarbide-derived carbon Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2). ...
s (CDCs) or carbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon nan ...
s (CNTs) as electrodes provides a network of small pores formed by nanotube entanglement. These nanoporous
Nanoporous materials consist of a regular organic or inorganic bulk phase in which a porous structure is present. Nanoporous materials exhibit pore diameters that are most appropriately quantified using units of nanometers. The diameter of pores i ...
materials have diameters in the range of <2 nm that can be referred to as intercalated pores. Solvated ions in the electrolyte are unable to enter these small pores, but de-solvated ions that have reduced their ion dimensions are able to enter, resulting in larger ionic packing density and increased charge storage. The tailored sizes of pores in nano-structured carbon electrodes can maximize ion confinement, increasing specific capacitance by faradaic adsorption treatment. Occupation of these pores by de-solvated ions from the electrolyte solution occurs according to (faradaic) intercalation.A.G. Pandolfo, A.F. HollenkampCarbon properties and their role in supercapacitors
, Journal of Power Sources 157 (2006) 11–27B.P. Bakhmatyuk, B.Ya. Venhryn, I.I. Grygorchak, M.M. Micov and S.I. Mudry
INTERCALATION PSEUDO-CAPACITANCE IN CARBON SYSTEMS OF ENERGY STORAGE
/ref>P. Simon, A. Burke
Nanostructured carbons: Double-Layer capacitance and more
Verification
 Pseudocapacitance properties can be expressed in a cyclic voltammogram. For an ideal double-layer capacitor, the current flow is reversed immediately upon reversing the potential yielding a rectangular-shaped voltammogram, with a current independent of the electrode potential. For double-layer capacitors with resistive losses, the shape changes to a
Pseudocapacitance properties can be expressed in a cyclic voltammogram. For an ideal double-layer capacitor, the current flow is reversed immediately upon reversing the potential yielding a rectangular-shaped voltammogram, with a current independent of the electrode potential. For double-layer capacitors with resistive losses, the shape changes to a parallelogram
In Euclidean geometry, a parallelogram is a simple (non- self-intersecting) quadrilateral with two pairs of parallel sides. The opposite or facing sides of a parallelogram are of equal length and the opposite angles of a parallelogram are of eq ...
. In faradaic electrodes the electrical charge stored in the capacitor is strongly dependent on the potential, therefore, the voltammetry characteristics deviate from the parallelogram due to a delay while reversing the potential, ultimately coming from kinetic charging processes.Elzbieta Frackowiak, Francois Beguin, PERGAMON, Carbon 39 (2001) 937–950Carbon materials for the electrochemical storage of energy in Capacitors
/ref>
/ref>
Applications
Pseudocapacitance is an important property insupercapacitor
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable ...
s.
Literature
* * * * * * *References
{{reflist, 2 Capacitors