|
Zinc Phosphide
Zinc phosphide ( Zn3 P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2). Synthesis and reactions Zinc phosphide can be prepared by the reaction of zinc with phosphorus; however, for critical applications, additional processing to remove arsenic compounds may be needed. :6 Zn + P4 → 2 Zn3P2 Another method of preparation include reacting tri-n-octylphosphine with dimethylzinc. Zinc phosphide reacts with water to produce phosphine (PH3) and zinc hydroxide (Zn(OH)2): :Zn3P2 + 6 H2O → 2 PH3 + 3 Zn(OH)2 Structure Zn3P2 has a room-temperature tetragonal form that converts to a cubic form at around 845 °C.Evgeniĭ I︠U︡rʹevich Tonkov, 1992, High Pressure Phase Transfor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.Solar Cells chemistryexplained.com It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as current, , or resistance, vary when exposed to light. Individual solar cell devices are often the electrical building blocks of [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Alberta
The University of Alberta, also known as U of A or UAlberta, is a Public university, public research university located in Edmonton, Alberta, Canada. It was founded in 1908 by Alexander Cameron Rutherford,"A Gentleman of Strathcona – Alexander Cameron Rutherford", Douglas R. Babcock, 1989, The University of Calgary Press, 2500 University Drive NW, Calgary, Alberta, Canada, the first premier of Alberta, and Henry Marshall Tory,"Henry Marshall Tory, A Biography", originally published 1954, current edition January 1992, E.A. Corbett, Toronto: Ryerson Press, the university's first president. It was enabled through the Post-secondary Learning Act''.'' The university is considered a "comprehensive academic and research university" (CARU), which means that it offers a range of academic and professional programs that generally lead to undergraduate and graduate level credentials. The university comprises four campuses in Edmonton, an Augustana Campus in Camrose, Alberta, Camrose, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Telluride
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell. Applications CdTe is used to make thin film solar cells, accounting for about 8% of all solar cells installed in 2011. They are among the lowest-cost types of solar cell, although a comparison of total installed cost depends on installation size and many other factors, and has changed rapidly from year to year. The CdTe solar cell market is dominated by First Solar. In 2011, around 2 GWp of CdTe solar cells were produced; For more details and discussion see cadmium telluride photovoltaics. CdTe can be alloyed with mercury to make a versatile infrared detector material ( HgCdTe). CdTe alloyed with a small amount of zinc makes an excellent solid-state X-ray and gamma ray detect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaics
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors. A photovoltaic system employs solar modules, each comprising a number of solar cells, which generate electrical power. PV installations may be ground-mounted, rooftop-mounted, wall-mounted or floating. The mount may be fixed or use a solar tracker to follow the sun across the sky. Photovoltaic technology helps to mitigate climate change because it emits much less carbon dioxide than fossil fuels. Solar PV has specific advantages as an energy source: once installed, its operation generates no pollution and no greenhouse gas emissions, it shows scalability in respect of power needs and silicon has large availability in the Earth's crust, although other materials required in PV sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cadmium Phosphide Arsenide
Zinc cadmium phosphide arsenide ( Zn- Cd- P- As) is a quaternary system of group II (IUPAC group 12) and group V (IUPAC group 15) elements. Many of the inorganic compounds in the system are II-V semiconductor materials. The quaternary system of II3V2 compounds, (Zn1−xCdx)3(P1−yAsy)2, has been shown to allow solid solution continuously over the whole compositional range. This material system and its subsets have applications in electronics, optoelectronics, including photovoltaics, and thermoelectrics. List of all binary compounds This system of elements contains numerous binary compounds and their solid solutions. Stable at atmospheric pressure The binary compounds thermodynamically stable at atmospheric pressure are listed in the following table: Metastable or unstable at atmospheric pressure Compounds metastable or unstable at atmospheric pressure are the following: Quaternary compounds The compounds of the form II3V2 have similar crystalline structures and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Phosphide
Cadmium phosphide ( Cd3 P2) is an inorganic chemical compound. It is a grey or white bluish solid semiconductor material with a bandgap of 0.5 eV. It has applications as a pesticide, material for laser diodes and for high-power-high-frequency electronics. Synthesis and reactions Cadmium phosphide can be prepared by the reaction of cadmium with phosphorus: :6 Cd + P4 → 2 Cd3P2 Structure Cd3P2 has a room-temperature tetragonal form. The crystalline structure of cadmium phosphide is very similar to that of zinc phosphide (Zn3P2), cadmium arsenide (Cd3As2) and zinc arsenide (Zn3As2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Applications Safety Like other metal phosphides, it is acutely toxic when swallowed due to the formation of phosphine gas when it reacts with gastric acid. It is also carcinogen and dangerous for the skin, eyes and other organs in a large part due to cadmium poisoning. References {{Phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Arsenide
Zinc arsenide (Zn3As2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is an inorganic semiconductor with a band gap of 1.0 eV. Synthesis and reactions Zinc arsenide can be prepared by the reaction of zinc with arsenic :3 Zn + 2 As → Zn3As2 Structure Zn3As2 has a room-temperature tetragonal form that converts to a different tetragonal phase at 190 °C and to a third phase at 651 °C. In the room-temperature form, the zinc atoms are tetrahedrally coordinated and the arsenic atoms are surrounded by six zinc atoms at the vertices of a distorted cube. The crystalline structure of zinc arsenide is very similar to that of cadmium arsenide (Cd3As2), zinc phosphide (Zn3P2) and cadmium phosphide (Cd3P2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Electronic structure Its lowest direct and indirect bandgaps are within 30 meV or each other. References arsenide zinc Zinc i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
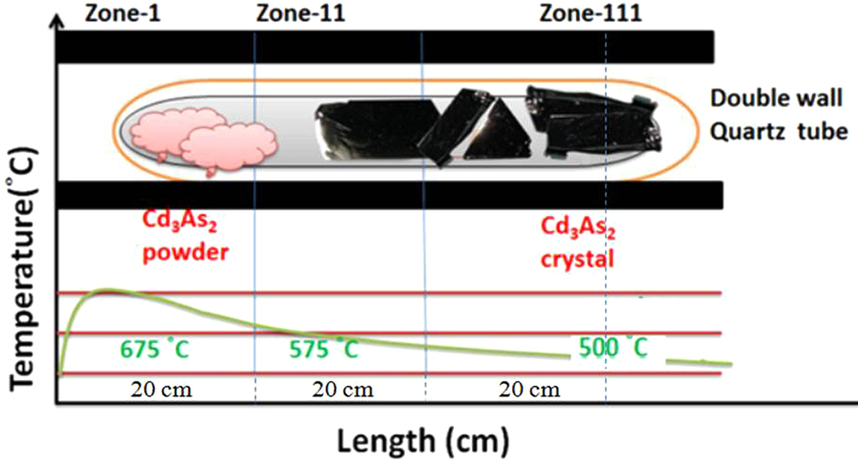
Cadmium Arsenide
Cadmium arsenide (cadmium, Cd3arsenic, As2) is an inorganic semimetal in the List of semiconductor materials#Types of semiconductor materials, II-V family. It exhibits the Nernst effect. Properties Thermal Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) An activation energy, energy barrier was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/ Phase transition Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal Crystal System
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |