cubic crystal system on:
[Wikipedia]
[Google]
[Amazon]

 In
In
 As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the orbital hybridization demands certain bond angles.) Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in polonium. The ''bcc'' and ''fcc'', with their higher densities, are both quite common in nature. Examples of ''bcc'' include iron,
As a rule, since atoms in a solid attract each other, the more tightly packed arrangements of atoms tend to be more common. (Loosely packed arrangements do occur, though, for example if the orbital hybridization demands certain bond angles.) Accordingly, the primitive cubic structure, with especially low atomic packing factor, is rare in nature, but is found in polonium. The ''bcc'' and ''fcc'', with their higher densities, are both quite common in nature. Examples of ''bcc'' include iron,
 One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
 It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the coordination number is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.Seitz, ''Modern Theory of Solids'' (1940), p.49 Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The space group of the
It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the coordination number is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.Seitz, ''Modern Theory of Solids'' (1940), p.49 Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The space group of the
 The space group of the rock-salt or halite (sodium chloride) structure is denoted as Fmm (in Hermann–Mauguin notation), or "225" (in the International Tables for Crystallography). The
The space group of the rock-salt or halite (sodium chloride) structure is denoted as Fmm (in Hermann–Mauguin notation), or "225" (in the International Tables for Crystallography). The
 The space group of the Zincblende structure is called F3m (in Hermann–Mauguin notation), or 216. The Strukturbericht designation is "B3".
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (
The space group of the Zincblende structure is called F3m (in Hermann–Mauguin notation), or 216. The Strukturbericht designation is "B3".
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (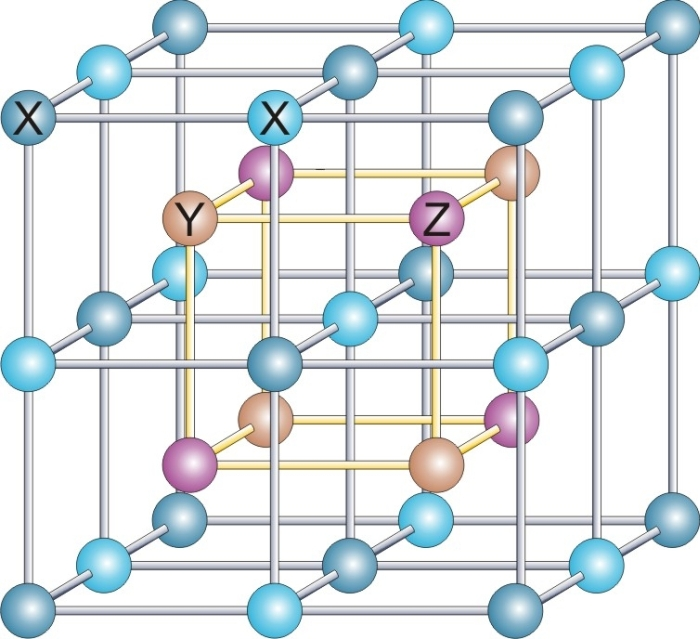
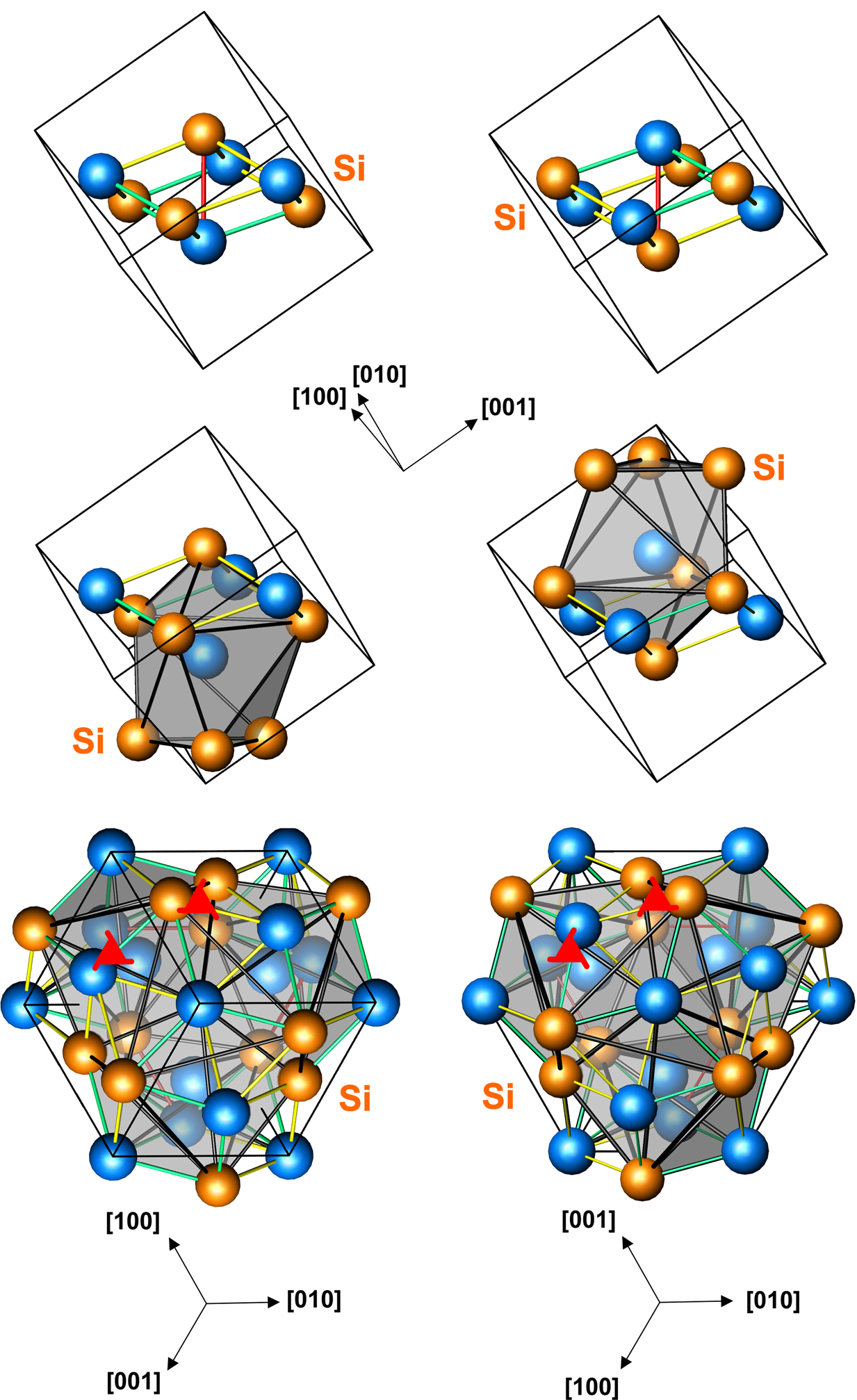 The space group of the iron monosilicide structure is P213 (No. 198), and the
The space group of the iron monosilicide structure is P213 (No. 198), and the
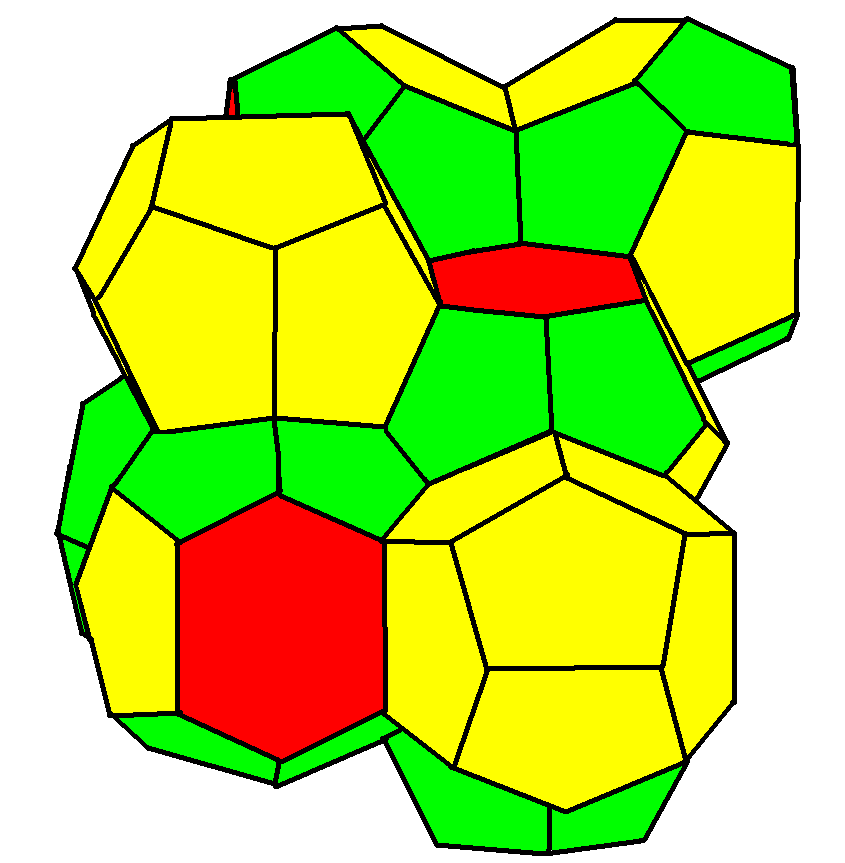 A Weaire–Phelan structure has Pmn (223) symmetry.
It has three orientations of stacked tetradecahedrons with pyritohedral cells in the gaps. It is found as a crystal structure in
A Weaire–Phelan structure has Pmn (223) symmetry.
It has three orientations of stacked tetradecahedrons with pyritohedral cells in the gaps. It is found as a crystal structure in
Simple cubic
BCC
FCC
HCP
Making crystal structure
with Molview {{DEFAULTSORT:Cubic Crystal System Crystal systems Cubes
crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube
In geometry, a cube is a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex. Viewed from a corner it is a hexagon and its net is usually depicted as a cross.
The cube is the only r ...
. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals:
*Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic)
*Body-centered cubic (abbreviated ''cI'' or bcc)
*Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp)
Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not.
Bravais lattices
The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes, and the unit cell therefore contains in total one atom ( × 8). The body-centered cubic lattice (cI) has one lattice point in the center of the unit cell in addition to the eight corner points. It has a net total of two lattice points per unit cell ( × 8 + 1). The face-centered cubic lattice (cF) has lattice points on the faces of the cube, that each gives exactly one half contribution, in addition to the corner lattice points, giving a total of 4 lattice points per unit cell ( × 8 from the corners plus × 6 from the faces). The face-centered cubic lattice is closely related to the hexagonal close packed (hcp) system, where two systems differ only in the relative placements of their hexagonal layers. The 11plane of a face-centered cubic lattice is a hexagonal grid. Attempting to create a base-centered cubic lattice (i.e., putting an extra lattice point in the center of each horizontal face) results in a simple tetragonal Bravais lattice. Coordination number (CN) is the number of nearest neighbors of a central atom in the structure. Each sphere in a cP lattice has coordination number 6, in a cI lattice 8, and in a cF lattice 12. Atomic packing factor (APF) is the fraction of volume that is occupied by atoms. The cP lattice has an APF of about 0.524 , the cI lattice an APF of about 0.680, and the cF lattice an APF of about 0.740.Crystal classes
The ''isometric crystal system'' class names, point groups (in Schönflies notation, Hermann–Mauguin notation, orbifold, andCoxeter notation
In geometry, Coxeter notation (also Coxeter symbol) is a system of classifying symmetry groups, describing the angles between fundamental reflections of a Coxeter group in a bracketed notation expressing the structure of a Coxeter-Dynkin diagr ...
), type, examples, international tables for crystallography space group number, and space groups are listed in the table below. There are a total 36 cubic space groups.
Other terms for hexoctahedral are: normal class, holohedral, ditesseral central class, galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
type.
Single element structures
chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
, tungsten, and niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
. Examples of ''fcc'' include aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
, gold and silver.
Another important cubic crystal structure is the diamond cubic structure, which can appear in carbon, silicon, germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, and tin. Unlike fcc and bcc, this structure is not a lattice, since it contains multiple atoms in its primitive cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
. Other cubic elemental structures include the A15 structure found in tungsten, and the extremely complicated structure of manganese.
Multi-element structures
Compounds that consist of more than one element (e.g. binary compounds) often have crystal structures based on the cubic crystal system. Some of the more common ones are listed here. These structures can be viewed as two or more interpenetrating sublattices where each sublattice occupies the interstitial sites of the others.Caesium chloride structure
 One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
One structure is the "interpenetrating primitive cubic" structure, also called a "caesium chloride" structure. This structure is often confused for a body-centered cubic structure, because the arrangement of atoms is the same. The true structure is shown in the graphic showing two individual primitive cubic structures that are superimposed within each other with the corner of one structure in the center of the cube of the other structure. It helps to convince yourself that it is not body-centered cubic because there is no translational symmetry along the ½, ½, ½, plane, the chloride would be translated into a cesium, not another chloride.
 It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the coordination number is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.Seitz, ''Modern Theory of Solids'' (1940), p.49 Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The space group of the
It works the same way for the NaCl structure described in the next section. If you take out the Cl atoms, the leftover Na atoms still form an FCC structure, not a simple cubic structure.
In the unit cell of CsCl, each ion is at the center of a cube of ions of the opposite kind, so the coordination number is eight. The central cation is coordinated to 8 anions on the corners of a cube as shown, and similarly, the central anion is coordinated to 8 cations on the corners of a cube. Alternately, one could view this lattice as a simple cubic structure with a secondary atom in its cubic void.
In addition to caesium chloride itself, the structure also appears in certain other alkali halides when prepared at low temperatures or high pressures.Seitz, ''Modern Theory of Solids'' (1940), p.49 Generally, this structure is more likely to be formed from two elements whose ions are of roughly the same size (for example, ionic radius of Cs+ = 167 pm, and Cl− = 181 pm).
The space group of the caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
(CsCl) structure is called Pmm (in Hermann–Mauguin notation), or "221" (in the International Tables for Crystallography). The Strukturbericht designation In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as suppl ...
is "B2".
There are nearly a hundred rare earth intermetallic compounds that crystalize in the CsCl structure, including many binary compounds of rare earths with magnesium, and with elements in groups 11, 12, and 13. Other compounds showing caesium chloride like structure are CsBr, CsI, high-temperature RbCl, AlCo, AgZn, BeCu, MgCe, RuAl and SrTl.
Rock-salt structure
Strukturbericht designation In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as suppl ...
is "B1".
In the rock-salt structure, each of the two atom types forms a separate face-centered cubic lattice, with the two lattices interpenetrating so as to form a 3D checkerboard pattern. The rock-salt structure has octahedral coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a centr ...
: Each atom's nearest neighbors consist of six atoms of the opposite type, positioned like the six vertices of a regular octahedron. In sodium chloride there is a 1:1 ratio of sodium to chlorine atoms. The structure can also be described as an FCC lattice of sodium with chlorine occupying each octahedral void or vice versa.
Examples of compounds with this structure include sodium chloride itself, along with almost all other alkali halides, and "many divalent metal oxides, sulfides, selenides, and tellurides". According to the radius ratio rule, this structure is more likely to be formed if the cation is somewhat smaller than the anion (a cation/anion radius ratio of 0.414 to 0.732).
The interatomic distance (distance between cation and anion, or half the unit cell length ''a'') in some rock-salt-structure crystals are: 2.3 Å (2.3 × 10−10 m) for NaF, 2.8 Å for NaCl, and 3.2 Å for SnTe. Most of the alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
hydrides and halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s have the rock salt structure, though a few have the caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
structure instead.
Many transition metal monoxides also have the rock salt structure (TiO
Titanium(II) oxide ( Ti O) is an inorganic chemical compound of titanium and oxygen. It can be prepared from titanium dioxide and titanium metal at 1500 °C. It is non-stoichiometric in a range TiO0.7 to TiO1.3 and this is caused by vacancie ...
, VO, CrO, MnO
Manganese(II) oxide is an inorganic compound with chemical formula MnO.Arno H. Reidies "Manganese Compounds" Ullmann's Encyclopedia of Chemical Technology 2007; Wiley-VCH, Weinheim. It forms green crystals. The compound is produced on a large ...
, FeO, CoO, NiO, CdO). The early actinoid monocarbides also have this structure ( ThC, PaC Pac or PAC may refer to:
Military
* Rapid Deployment Force (Malaysia), an armed forces unit
* Patriot Advanced Capability, of the MIM-104 Patriot missile
* Civil Defense Patrols (''Patrullas de Autodefensa Civil''), Guatemalan militia and paramili ...
, UC, NpC, PuC
PUC or P.U.C. may refer to:
Education
* Pacific Union College
* Pre-university course
* Pentecost University College
* Premier University, Chittagong
* Pontifical Catholic University (from Pontificia Universidad(e) Católica)
** Pontifi ...
). Other compounds showing rock salt like structure are TiB, ZrB, PbS, PbSe, PbTe, SnTe, AgF, AgCl, and AgBr.
Fluorite structure
Much like the rock salt structure, the fluorite structure (AB2) is also an Fmm structure but has 1:2 ratio of ions. The anti-fluorite structure is nearly identical, except the positions of the anions and cations are switched in the structure. They are designated Wyckoff positions 4a and 8c whereas the rock-salt structure positions are 4a and 4b.Zincblende structure
 The space group of the Zincblende structure is called F3m (in Hermann–Mauguin notation), or 216. The Strukturbericht designation is "B3".
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (
The space group of the Zincblende structure is called F3m (in Hermann–Mauguin notation), or 216. The Strukturbericht designation is "B3".
The Zincblende structure (also written "zinc blende") is named after the mineral zincblende (sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimen ...
), one form of zinc sulfide (β-ZnS). As in the rock-salt structure, the two atom types form two interpenetrating face-centered cubic lattices. However, it differs from rock-salt structure in how the two lattices are positioned relative to one another. The zincblende structure has tetrahedral coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a centr ...
: Each atom's nearest neighbors consist of four atoms of the opposite type, positioned like the four vertices of a regular tetrahedron. In zinc sulfide the ratio of zinc to sulfur is 1:1. Altogether, the arrangement of atoms in zincblende structure is the same as diamond cubic structure, but with alternating types of atoms at the different lattice sites. The structure can also be described as an FCC lattice of zinc with sulfur atoms occupying half of the tetrahedral voids or vice versa.
Examples of compounds with this structure include zincblende itself, lead(II) nitrate, many compound semiconductors (such as gallium arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure.
Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monoli ...
and cadmium telluride), and a wide array of other binary compounds. The boron group pnictogenides usually have a zincblende structure, though the nitrides are more common in the wurtzite structure, and their zincblende forms are less well known polymorph
Polymorphism, polymorphic, polymorph, polymorphous, or polymorphy may refer to:
Computing
* Polymorphism (computer science), the ability in programming to present the same programming interface for differing underlying forms
* Ad hoc polymorphi ...
s.
This group is also known as the II-VI family of compounds, most of which can be made in both the zincblende (cubic) or wurtzite (hexagonal) form.
This group is also known as the III-V family of compounds.

Heusler structure
The Heusler structure, based on the structure of Cu2MnAl, is a common structure for ternary compounds involving transition metals. It has the space group Fmm (No. 225), and theStrukturbericht designation In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as suppl ...
is L21. Together with the closely related half-Heusler and inverse-Huesler compounds, there are hundreds of examples.
Iron monosilicide structure
 The space group of the iron monosilicide structure is P213 (No. 198), and the
The space group of the iron monosilicide structure is P213 (No. 198), and the Strukturbericht designation In crystallography, a Strukturbericht designation or Strukturbericht type is a system of detailed crystal structure classification by analogy to another known structure. The designations were intended to be comprehensive but are mainly used as suppl ...
is B20. This is a chiral structure, and is sometimes associated with helimagnetic properties. There are four atoms of each element for a total of eight atoms in the unit cell.
Examples occur among the transition metal silicides and germanides, as well as a few other compounds such as gallium palladide
Gallium palladide (GaPd or PdGa) is an intermetallic combination of gallium and palladium. In the Iron monosilicide crystal structure. The compound has been suggested as an improved Hydrogenation#Catalysts, catalyst for hydrogenation reactions. In ...
.
Weaire–Phelan structure
 A Weaire–Phelan structure has Pmn (223) symmetry.
It has three orientations of stacked tetradecahedrons with pyritohedral cells in the gaps. It is found as a crystal structure in
A Weaire–Phelan structure has Pmn (223) symmetry.
It has three orientations of stacked tetradecahedrons with pyritohedral cells in the gaps. It is found as a crystal structure in chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
where it is usually known as a "type I clathrate structure". Gas hydrates formed by methane, propane, and carbon dioxide at low temperatures have a structure in which water molecules lie at the nodes of the Weaire–Phelan structure and are hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ed together, and the larger gas molecules are trapped in the polyhedral cages.
See also
* Atomium: building which is a model of a ''bcc'' unit cell, with vertical body diagonal. * Close-packing * Dislocations * Reciprocal latticeReferences
Further reading
*Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., Wiley,External links
* JMol simulations byGraz University
The University of Graz (german: link=no, Karl-Franzens-Universität Graz, ), located in Graz, Austria, is the largest and oldest university in Styria, as well as the second-largest and second-oldest university in Austria.
History
The unive ...
:
Simple cubic
BCC
FCC
HCP
Making crystal structure
with Molview {{DEFAULTSORT:Cubic Crystal System Crystal systems Cubes