|
Helimagnetic
Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature. Strictly speaking, helimagnets have no permanent magnetic moment, and as such are sometimes considered a complicated type of antiferromagnet. This distinguishes helimagnets from conical magnets, (e.g. Holmium below 20 K) which have spiral modulation in addition to a permanent magnetic moment. Helimagnetism was first proposed in 1959, as an explanation of the magnetic structure of manganese dioxide. Initially a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Structure
The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics. Magnetic structures Most solid materials are non-magnetic, that is, they do not display a magnetic structure. Due to the Pauli exclusion principle, each state is occupied by electrons of opposing spins, so that the charge density is compensated everywhere and the spin degree of freedom is trivial. Still, such materials typically do show a weak magnetic behaviour, e.g. due to Pauli paramagnetism or Langevin or Landau diamagnetism. The more interesting case is when the material's electron spontaneously break above-mentioned symmetry. For ferromagnetism in the ground state, there is a common spin quantization axis and a global excess of electrons of a given spin quantum number, there are more electrons pointing in one direction than in the other, giving a macroscopic magnetization (typical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Phosphide
Iron phosphide is a chemical compound of iron and phosphorus, with a formula of FeP. Its physical appearance is grey, hexagonal needles. Manufacturing of iron phosphide takes place at elevated temperatures, where the elements combine directly. Iron phosphide reacts with moisture and acids producing phosphine (PH3), a toxic and pyrophoric gas. Iron phosphide can be used as a semiconductor. It has use in high power, high frequency applications, such as laser diodes. Below a Néel temperature of about 119 K, FeP takes on an helimagnetic structure. Hazards and mitigation Iron phosphide is a hazardous substance. Proper eye protection such as goggles should always be used when handling iron phosphide. It can be very harmful to the eyes, especially for individuals wearing contact lenses Contact lenses, or simply contacts, are thin lenses placed directly on the surface of the eyes. Contact lenses are ocular prosthetic devices used by over 150 million people worldwide, and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
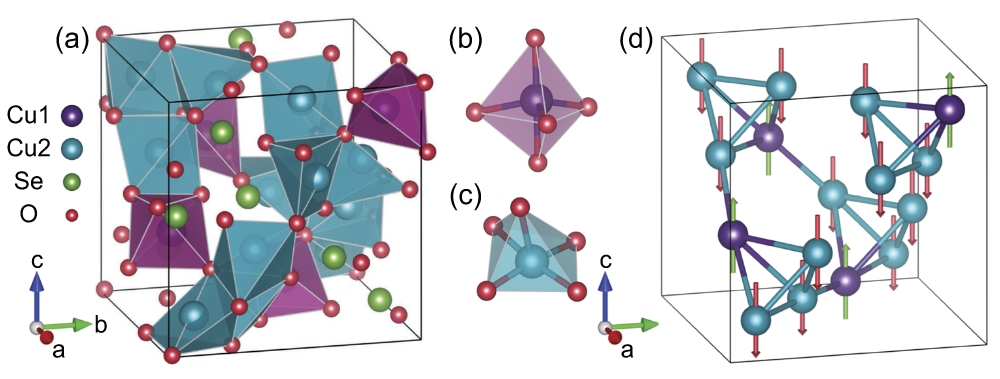
Copper(I) Oxide Selenite
Copper oxide selenite is an inorganic compound with the chemical formula Cu2OSeO3. It is an electrically insulating, piezoelectric and piezomagnetic material, which becomes a ferrimagnet upon cooling below 58 K. As of 2021, Cu2OSeO3 is the only insulating material that hosts magnetic skyrmions. Synthesis Cu2OSeO3 polycrystals can be grown by heating a 2:1 molar mixture of CuO and SeO2 powders at 600 °C for 12 hours in vacuum. They can be converted into olive-green single crystals ca. 4 mm in size by chemical vapor transport. NH4Cl is used as the transport agent; it sublimes at 340 °C, yielding NH3 and HCl gases. Structure Cu2OSeO3 crystals have a cubic, distorted pyrochlore structure built by Cu4O and SeO3 units. The spins on three Cu2+ ions in each tetrahedron (Cu1 sites) are aligned, while the Cu2 spin is facing in the opposite direction, resulting in a ferrimagnetic order. The helical spin and skyrmion textures emerge at low magnetic fields due to the Dzyaloshinskii-Moriya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Monosilicide
Manganese monosilicide (MnSi) is an intermetallic compound, a silicide of manganese. It occurs in cosmic dust as the mineral brownleeite. MnSi has a cubic crystal lattice with no inversion center; therefore its crystal structure is helical, with right-hand and left-hand chiralities. MnSi is a paramagnetic metal that turns into a ferromagnet at cryogenic temperatures below 29 K. In the ferromagnetic state, the spatial arrangement of electron spins in MnSi changes with magnetic field, forming helical, conical, skyrmion, and regular ferromagnetic phases. Crystal structure and magnetism Manganese monosilicide is a non-stoichiometric compound, meaning that the 1:1 Mn:Si composition, lattice constant and many other properties vary depending on the synthesis and processing history of the crystal. MnSi has a cubic crystal lattice with no inversion center; therefore its crystal structure is helical, with right-hand and left-hand chiralities. At low temperatures and magnetic fields, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Germanide
Iron germanide (FeGe) is an intermetallic compound, a germanide of iron. At ambient conditions it crystallizes in three Polymorphism (materials science), polymorphs with monoclinic, hexagonal and cubic structures. The cubic polymorph has no inversion center, it is therefore helical, with right-hand and left-handed Chirality, chiralities. Magnetism FeGe is extensively studied for its unusual magnetic properties. Electron spins in this material show dissimilar, yet regular spatial arrangements at different values of applied magnetic field. Those arrangements are named Helimagnetism, helical, Magnetic skyrmion, skyrmion lattice, and conical. They can be controlled not only by temperature and magnetic field, but also by electric current, and the current density required for manipulating skyrmions (~106 A/m2) is approximately one million times smaller than that needed for moving magnetic domains in traditional ferromagnets. As a result, skyrmions have potential application in ultrahigh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetic Resonance
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization (Larmor precession) of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization (Larmor frequency) and the polarization of the wave must match the orientation of the magnetization. This effect can be used for various applications such as spectroscopic techniques or conception of microwave devices. The FMR spectroscopic technique is used to probe the magnetization of ferromagnetic materials. It is a standard tool for probing spin waves and spin dynamics. FMR is very broadly similar to electron paramagnetic resonance (EPR), and also somewhat similar to nuclear magnetic resonance (NMR), except that FMR probes the sample magnetization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Skyrmion
In physics, magnetic skyrmions (occasionally described as 'vortices,' or 'vortex-like' configurations) are statically stable solitons which have been predicted theoretically and observed experimentally in condensed matter systems. Skyrmions can be formed in magnetic materials in their 'bulk' such as in MnSi, or in magnetic thin films. They can be achiral, or chiral (Fig. 1 a and b are both chiral skyrmions) in nature, and may exist both as dynamic excitations or stable or metastable states. Although the broad lines defining magnetic skyrmions have been established de facto, there exist a variety of interpretations with subtle differences. Most descriptions include the notion of topology – a categorization of shapes and the way in which an object is laid out in space – using a continuous-field approximation as defined in micromagnetics. Descriptions generally specify a non-zero, integer value of the topological index, (not to be confused with the chemistry meaning of 'topologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antisymmetric Exchange
In Physics, antisymmetric exchange, also known as the Dzyaloshinskii–Moriya interaction (DMI), is a contribution to the total magnetic exchange interaction between two neighboring magnetic spins, \mathbf_i and \mathbf_j . Quantitatively, it is a term in the Hamiltonian which can be written as : H^_=\mathbf_ \cdot ( \mathbf_i \times \mathbf_j ). In magnetically ordered systems, it favors a spin canting of otherwise parallel or antiparallel aligned magnetic moments and thus, is a source of weak ferromagnetic behavior in an antiferromagnet. The interaction is fundamental to the production of magnetic skyrmions and explains the magnetoelectric effects in a class of materials termed multiferroics. History The discovery of antisymmetric exchange originated in the early 20th century from the controversial observation of weak ferromagnetism in typically antiferromagnetic -FeO crystals. In 1958, Igor Dzyaloshinskii provided evidence that the interaction was due to the relativistic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holmium
Holmium is a chemical element with the symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like a lot of other lanthanides, holmium is too reactive to be found in native form, as pure holmium slowly forms a yellowish oxide coating when exposed to air. When isolated, holmium is relatively stable in dry air at room temperature. However, it reacts with water and corrodes readily, and also burns in air when heated. In nature, holmium occurs together with the other rare-earth metals (like thulium). It is a relatively rare lanthanide, making up 1.4 parts per million of the Earth's crust, an abundance similar to tungsten. Holmium was discovered through isolation by Swedish chemist Per Theodor Cleve and independently by Jacques-Louis Soret and Marc Delafontaine, who observed it spectroscopically in 1878. Its oxide was first isolated from rare-earth ores ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dysprosium
Dysprosium is the chemical element with the symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy. Dysprosium was first identified in 1886 by Paul Émile Lecoq de Boisbaudran, but it was not isolated in pure form until the development of ion-exchange techniques in the 1950s. Dysprosium has relatively few applications where it cannot be replaced by other chemical elements. It is used for its high thermal neutron absorption cross-section in making control rods in nuclear reactors, for its high magnetic susceptibility () in data-storage applications, and as a component of Terfenol-D (a magnetostrictive material). Soluble dysprosium salts are mildly toxic, while the insoluble salts are consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite. Swedish chemist Carl Gustaf Mosander discovered terbium as a chemical element in 1843. He detected it as an impurity in yttrium oxide, . Yttrium and terbium, as well as erbium and ytterbium, are named after the village of Ytterby in Sweden. Terbium was not isolated in pure form until the advent of ion exchange techniques. Terbium is used to dope calcium fluoride, calcium tungstate and strontium molybdate in solid-state devices, and as a crystal stabilizer of fuel cells that operate at elevated temperatures. As a component of Terfenol-D (an alloy that expands ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |