Copper(I) Oxide Selenite on:
[Wikipedia]
[Google]
[Amazon]
 Cu2OSeO3 polycrystals can be grown by heating a 2:1 molar mixture of
Cu2OSeO3 polycrystals can be grown by heating a 2:1 molar mixture of
 Cu2OSeO3 is a ferrimagnet, and all its properties below the
Cu2OSeO3 is a ferrimagnet, and all its properties below the
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
selenite
Selenite may refer to:
Substances containing selenium
*A selenium-containing anion or ionic compound with the SeO32− anion:
**Selenite (ion), anion is a selenium oxoanion with the chemical formula SeO32−
***Selenous acid, the conjugate acid, w ...
is an inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
Cu2OSeO3. It is an electrically insulating, piezoelectric
Piezoelectricity (, ) is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied Stress (mechanics), mechanical s ...
and piezomagnetic material, which becomes a ferrimagnet
A ferrimagnetic material is a material that has populations of atoms with opposing magnetic moments, as in antiferromagnetism, but these moments are unequal in magnitude so a spontaneous magnetization remains. This can for example occur when t ...
upon cooling below 58 K. As of 2021, Cu2OSeO3 is the only insulating material that hosts magnetic skyrmion
In physics, magnetic skyrmions (occasionally described as 'vortices,' or 'vortex-like'
configurations) are statically stable solitons which have been predicted theoretically and observed experimentally in condensed matter systems. Skyrmions can be ...
s.
Synthesis
 Cu2OSeO3 polycrystals can be grown by heating a 2:1 molar mixture of
Cu2OSeO3 polycrystals can be grown by heating a 2:1 molar mixture of CuO
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is ...
and SeO2 powders at 600 °C for 12 hours in vacuum. They can be converted into olive-green single crystals ca. 4 mm in size by chemical vapor transport
In chemistry, a chemical transport reaction describes a process for purification and crystallization of non- volatile solids. The process is also responsible for certain aspects of mineral growth from the effluent of volcanoes. The technique ...
. NH4Cl is used as the transport agent; it sublimes at 340 °C, yielding NH3 and HCl gases.
Structure
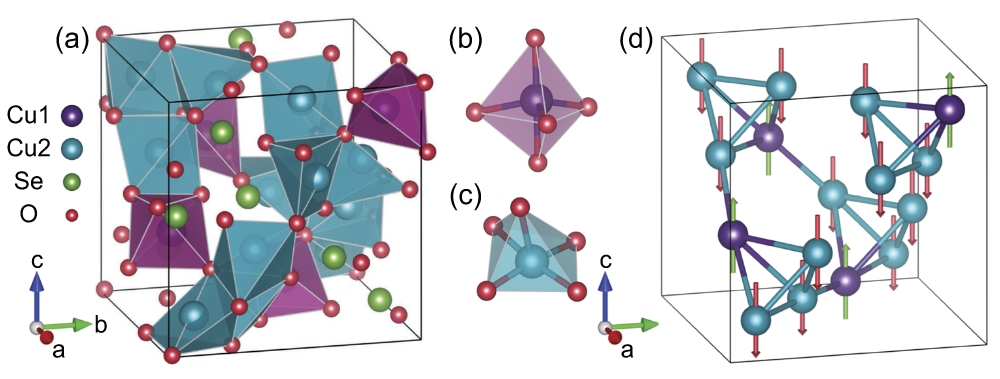
Cu2OSeO3 crystals have a cubic, distortedpyrochlore
Pyrochlore () is a mineral group of the niobium end member of the pyrochlore supergroup.
The general formula, (where A and B are metals), represent a family of phases isostructural to the mineral pyrochlore.
Pyrochlores are an important class of ...
structure built by Cu4O and SeO3 units. The spins on three Cu2+ ions in each tetrahedron (Cu1 sites) are aligned, while the Cu2 spin is facing in the opposite direction, resulting in a ferrimagnetic order. The helical spin and skyrmion textures emerge at low magnetic fields due to the Dzyaloshinskii-Moriya interaction
In Physics, antisymmetric exchange, also known as the Dzyaloshinskii–Moriya interaction (DMI), is a contribution to the total magnetic exchange interaction between two neighboring magnetic spins, \mathbf_i and \mathbf_j . Quantitatively, it i ...
.
Properties
 Cu2OSeO3 is a ferrimagnet, and all its properties below the
Cu2OSeO3 is a ferrimagnet, and all its properties below the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
strongly depend on magnetic field. With increasing field, its spin texture changes from helical stripes to conical stripes or skyrmion
In particle theory, the skyrmion () is a topologically stable field configuration of a certain class of non-linear sigma models. It was originally proposed as a model of the nucleon by (and named after) Tony Skyrme in 1961. As a topological solito ...
lattice, and then to a "field polarized", i.e., ferrimagnetic alignment. Thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
peaks around 9 K with a value of ca. 400 W/(m·K). The magnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Di ...
damping constant is 1 at 5 K. This value is only 4 times larger than that of yttrium iron garnet
Yttrium iron garnet (YIG) is a kind of synthetic garnet, with chemical composition , or Y3Fe5O12. It is a ferrimagnetic material with a Curie temperature of 560 K. YIG may also be known as yttrium ferrite garnet, or as iron yttrium oxide or ...
, which has the lowest magnetization damping value among all materials. This property is advantageous for high-frequency electronic applications, as it results in low current-induced heat.
References
{{Copper compounds Selenites Copper(I) compounds