|
Cadmium Arsenide
Cadmium arsenide ( Cd3 As2) is an inorganic semimetal in the II-V family. It exhibits the Nernst effect. Properties Thermal Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) An energy barrier was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/ Phase transition Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis loop. :α”-Cd3As2 ↔ β-Cd3As2 occurs at 868 K. Single crystal x-ray diffraction was used to dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scanning Tunneling Microscopy
A scanning tunneling microscope (STM) is a type of microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in Physics in 1986. STM senses the surface by using an extremely sharp conducting tip that can distinguish features smaller than 0.1 nm with a 0.01 nm (10 pm) depth resolution. This means that individual atoms can routinely be imaged and manipulated. Most microscopes are built for use in ultra-high vacuum at temperatures approaching zero kelvin, but variants exist for studies in air, water and other environments, and for temperatures over 1000 °C. STM is based on the concept of quantum tunneling. When the tip is brought very near to the surface to be examined, a bias voltage applied between the two allows electrons to tunnel through the vacuum separating them. The resulting ''tunneling current'' is a function of the tip position, applied ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Narrow-gap Semiconductor
Narrow-gap semiconductors are semiconducting materials with a band gap that is comparatively small compared to that of silicon, i.e. smaller than 1.11 eV at room temperature. They are used as infrared detectors or thermoelectrics. List of narrow-gap semiconductors : See also * List of semiconductor materials Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of ... * Wide-bandgap semiconductor References * Dornhaus, R., Nimtz, G., Schlicht, B. (1983). ''Narrow-Gap Semiconductors''. Springer Tracts in Modern Physics 98, (print) (online) * Semiconductor material types {{CMP-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Cadmium Phosphide Arsenide
Zinc cadmium phosphide arsenide ( Zn- Cd- P- As) is a quaternary system of group II (IUPAC group 12) and group V (IUPAC group 15) elements. Many of the inorganic compounds in the system are II-V semiconductor materials. The quaternary system of II3V2 compounds, (Zn1−xCdx)3(P1−yAsy)2, has been shown to allow solid solution continuously over the whole compositional range. This material system and its subsets have applications in electronics, optoelectronics, including photovoltaics, and thermoelectrics. List of all binary compounds This system of elements contains numerous binary compounds and their solid solutions. Stable at atmospheric pressure The binary compounds thermodynamically stable at atmospheric pressure are listed in the following table: Metastable or unstable at atmospheric pressure Compounds metastable or unstable at atmospheric pressure are the following: Quaternary compounds The compounds of the form II3V2 have similar crystalline structures and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Phosphide
Cadmium phosphide ( Cd3 P2) is an inorganic chemical compound. It is a grey or white bluish solid semiconductor material with a bandgap of 0.5 eV. It has applications as a pesticide, material for laser diodes and for high-power-high-frequency electronics. Synthesis and reactions Cadmium phosphide can be prepared by the reaction of cadmium with phosphorus: :6 Cd + P4 → 2 Cd3P2 Structure Cd3P2 has a room-temperature tetragonal form. The crystalline structure of cadmium phosphide is very similar to that of zinc phosphide (Zn3P2), cadmium arsenide (Cd3As2) and zinc arsenide (Zn3As2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Applications Safety Like other metal phosphides, it is acutely toxic when swallowed due to the formation of phosphine gas when it reacts with gastric acid. It is also carcinogen and dangerous for the skin, eyes and other organs in a large part due to cadmium poisoning. References {{Phospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Arsenide
Zinc arsenide (Zn3As2) is a binary compound of zinc with arsenic which forms gray tetragonal crystals. It is an inorganic semiconductor with a band gap of 1.0 eV. Synthesis and reactions Zinc arsenide can be prepared by the reaction of zinc with arsenic :3 Zn + 2 As → Zn3As2 Structure Zn3As2 has a room-temperature tetragonal form that converts to a different tetragonal phase at 190 °C and to a third phase at 651 °C. In the room-temperature form, the zinc atoms are tetrahedrally coordinated and the arsenic atoms are surrounded by six zinc atoms at the vertices of a distorted cube. The crystalline structure of zinc arsenide is very similar to that of cadmium arsenide (Cd3As2), zinc phosphide (Zn3P2) and cadmium phosphide (Cd3P2). These compounds of the Zn-Cd-P-As quaternary system exhibit full continuous solid-solution. Electronic structure Its lowest direct and indirect bandgaps are within 30 meV or each other. References arsenide zinc Zinc i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Phosphide
Zinc phosphide ( Zn3 P2) is an inorganic chemical compound. It is a grey solid, although commercial samples are often dark or even black. It is used as a rodenticide. Zn3P2 is a II-V semiconductor with a direct band gap of 1.5 eV and may have applications in photovoltaic cells. A second compound exists in the zinc-phosphorus system, zinc diphosphide (ZnP2). Synthesis and reactions Zinc phosphide can be prepared by the reaction of zinc with phosphorus; however, for critical applications, additional processing to remove arsenic compounds may be needed. :6 Zn + P4 → 2 Zn3P2 Another method of preparation include reacting tri-n-octylphosphine with dimethylzinc. Zinc phosphide reacts with water to produce phosphine (PH3) and zinc hydroxide (Zn(OH)2): :Zn3P2 + 6 H2O → 2 PH3 + 3 Zn(OH)2 Structure Zn3P2 has a room-temperature tetragonal form that converts to a cubic form at around 845 °C.Evgeniĭ I︠U︡rʹevich Tonkov, 1992, High Pressure Phase Transformati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Close-packing Of Equal Spheres
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by T. C. Hales. Highest density is known only for 1, 2, 3, 8, and 24 dimensions. Many crystal structures are based on a close-packing of a single kind of atom, or a close-packing of large ions with smaller ions filling the spaces between them. The cubic and hexagonal arrangements are very close to one anoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ingot
An ingot is a piece of relatively pure material, usually metal, that is cast into a shape suitable for further processing. In steelmaking, it is the first step among semi-finished casting products. Ingots usually require a second procedure of shaping, such as cold/hot working, cutting, or milling to produce a useful final product. Non-metallic and semiconductor materials prepared in bulk form may also be referred to as ingots, particularly when cast by mold based methods. Precious metal ingots can be used as currency (with or without being processed into other shapes), or as a currency reserve, as with gold bars. Types Ingots are generally made of metal, either pure or alloy, heated past its melting point and cast into a bar or block using a mold chill method. A special case are polycrystalline or single crystal ingots made by pulling from a molten melt. Single crystal Single crystal ingots (called boules) of materials are grown (crystal growth) using methods such as the Czo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material. Despite bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal. Etymology The term comes from the Greek ''a'' ("without"), and ''morphé'' ("shape, form"). In some older articles and books, the term was used synonymously with glass. Today, "glassy solid" or "amorphous solid" is considered the overarching concept. Polymers are often amorphous. Structure Amorphous materials have an internal structure comprising interconnected structural blocks that can be similar to the basic structural units found in the corresponding crystalline phase of the same compound. Unlike crystalline materials, however, no long-range order exists. Localized order in amorphous materials can be categorized as short or medium range order. By convention, short range order extends only to the nearest neighbor shell, typically only 1-2 atomic spacings. Medium range order is then de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
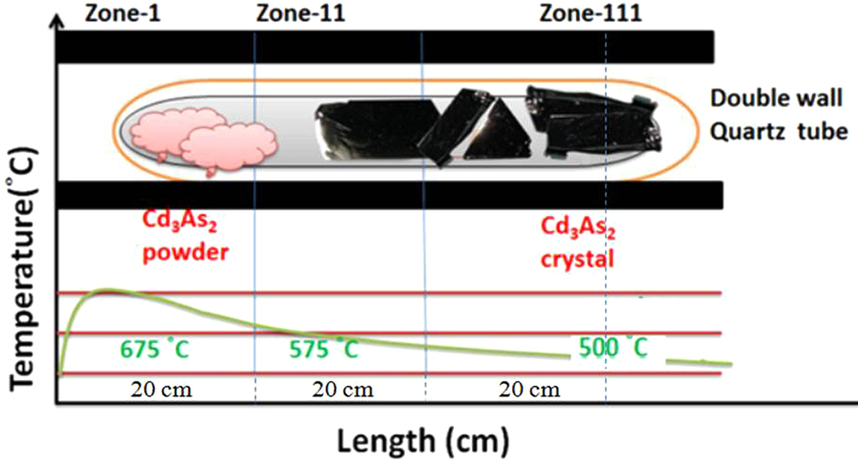
Cadmium Arsenide Growth
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than the transition metals in groups 3 through 11. Cadmium and its congeners in group 12 are often not considered transition metals, in that they do not have partly filled ''d'' or ''f'' electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate. Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, oran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Oscillations
In condensed matter physics, quantum oscillations describes a series of related experimental techniques used to map the Fermi surface of a metal in the presence of a strong magnetic field. These techniques are based on the principle of Landau quantization of Fermions moving in a magnetic field. For a gas of free fermions in a strong magnetic field, the energy levels are quantized into bands, called the ''Landau levels'', whose separation is proportional to the strength of the magnetic field. In a quantum oscillation experiment, the external magnetic field is varied, which causes the Landau levels to pass over the Fermi surface, which in turn results in oscillations of the electronic density of states at the Fermi level; this produces oscillations in the many material properties which depend on this, including resistance (the Shubnikov–de Haas effect), Hall resistance, and magnetic susceptibility (the de Haas–van Alphen effect). Observation of quantum oscillations in a mater ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |