|
Berkelium(III) Nitrate
Berkelium(III) nitrate is the berkelium salt of nitric acid with the formula Bk(NO3)3. It commonly forms the tetrahydrate, Bk(NO3)3·4H2O, which is a light green solid. If heated to 450 °C, it decomposes to berkelium(IV) oxide Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C. :2BkO2 + H2 → ... and 22 milligrams of the solution of this compound is reported to cost one million dollars. Production and uses Berkelium(III) nitrate is produced by the reaction of berkelium metal, the hydroxide, or chloride with nitric acid. This compound has no commercial uses, but was used to synthesize the element tennessine. The aqueous compound was painted onto a titanium foil and was bombarded with calcium-48 atoms to synthesize the element tennessine. This compound is used as a pathway to pentavalent berkel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digital Object Identifier
A digital object identifier (DOI) is a persistent identifier or handle used to uniquely identify various objects, standardized by the International Organization for Standardization (ISO). DOIs are an implementation of the Handle System; they also fit within the URI system ( Uniform Resource Identifier). They are widely used to identify academic, professional, and government information, such as journal articles, research reports, data sets, and official publications. DOIs have also been used to identify other types of information resources, such as commercial videos. A DOI aims to resolve to its target, the information object to which the DOI refers. This is achieved by binding the DOI to metadata about the object, such as a URL where the object is located. Thus, by being actionable and interoperable, a DOI differs from ISBNs or ISRCs which are identifiers only. The DOI system uses the indecs Content Model for representing metadata. The DOI for a document remains fixed over t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The production of the second-most important isotope, 247Bk, involves the irradiation of the rare isotope 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(IV) Oxide
Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C. :2BkO2 + H2 → Bk2O3 + H2O Production Berkelium(IV) oxide is produced by burning berkelium metal in air at 1200 °C. It can also be produced by reacting berkelium(III) oxide with oxygen at 600 °C. References {{Oxides Berkelium compounds Oxides Fluorite crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
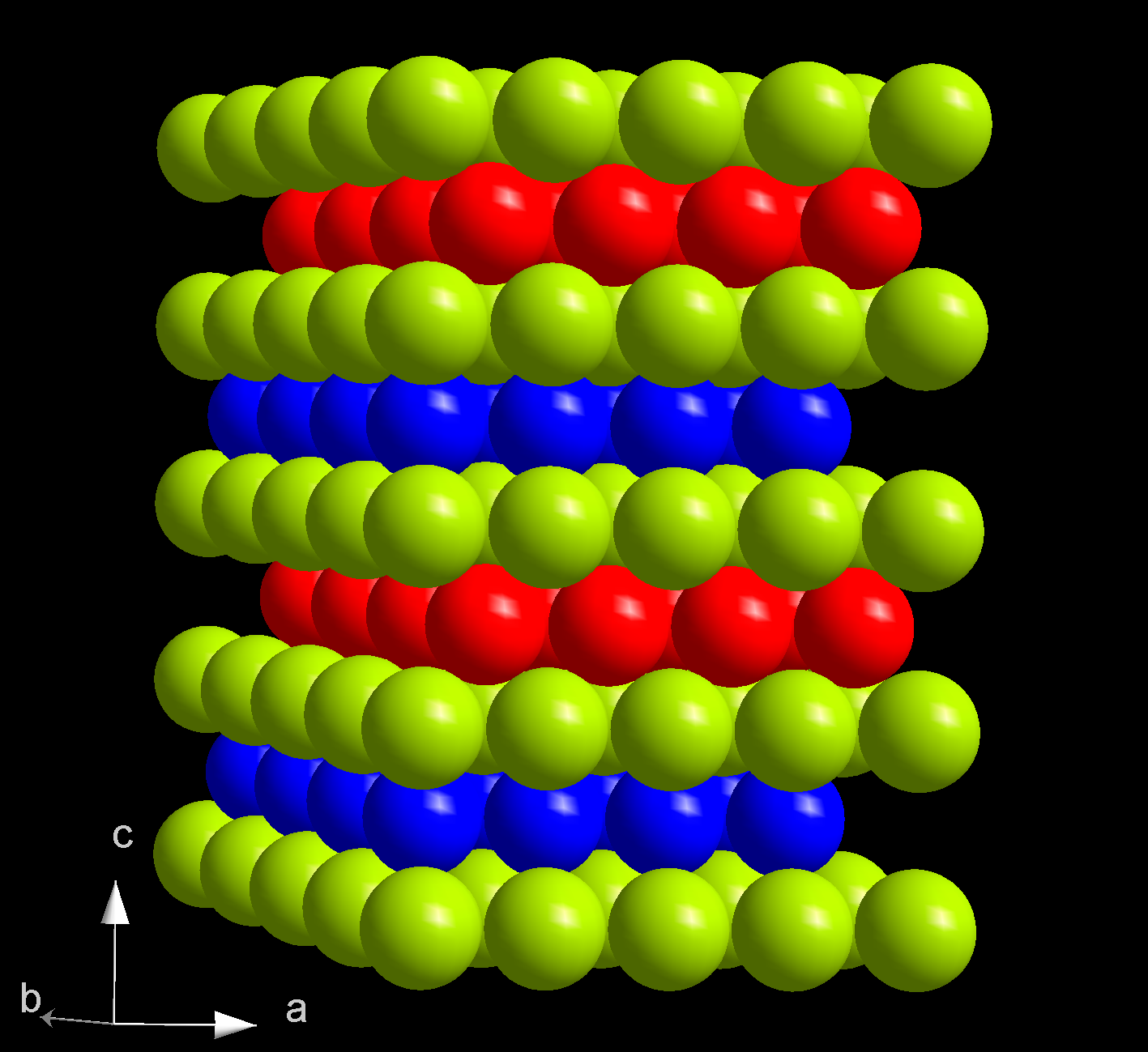
Berkelium(III) Chloride
Berkelium(III) chloride also known as berkelium trichloride, is a chemical compound with the formula BkCl3. It is a water-soluble green salt with a melting point of 603 °C. This compound forms the hexahydrate, BkCl3·6H2O. Preparation and reactions This compound was first prepared in 1970 by reacting hydrogen chloride gas and berkelium(IV) oxide or berkelium(III) oxide at 520 °C: :Bk2O3 + 6HCl → 2BkCl3 + 3H2O Berkelium(III) chloride reacts with beryllocene to produce berkelocene(Bk(C5H5)3). It also reacts with oxalic acid to produce berkelium oxalate. This reaction is used to purify this compound, by reacting the oxalate with hydrochloric acid. Structure Anhydrous berkelium(III) chloride has a hexagonal crystal structure, is isostructural to uranium trichloride, and has the person symbol hP6. When heated it its melting point, it converts to an orthorhombic phase. However, the hexahydrate has a monoclinic crystal structure and is isostructural to americium trichlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tennessine
Tennessine is a synthetic chemical element with the symbol Ts and atomic number 117. It is the second-heaviest known element and the penultimate element of the 7th period of the periodic table. The discovery of tennessine was officially announced in Dubna, Russia, by a Russian–American collaboration in April 2010, which makes it the most recently discovered element . One of its daughter isotopes was created directly in 2011, partially confirming the results of the experiment. The experiment itself was repeated successfully by the same collaboration in 2012 and by a joint German–American team in May 2014. In December 2015, the Joint Working Party of the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUPAP), which evaluates claims of discovery of new elements, recognized the element and assigned the priority to the Russian–American team. In June 2016, the IUPAC published a declaration stating that the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so the only radioactive decay pathway that it has been observed to undergo is the extremely rare process of double beta decay. Its half-life is about 6.4×1019 years, so for all practical purposes it can be treated as stable. One factor contributing to this unusual stability is that 20 and 28 are both magic numbers, making 48Ca a "doubly magic" nucleus. Since 48Ca is both practically stable and neutron-rich, it is a valuable starting material for the production of new nuclei in particle accelerators, both by fragmentation and by fusion reactions with other nuclei, for example in the discoveries of the heaviest five elements on the periodic table, from flerovium to oganesson. Heavier nuclei generally require a greater fraction of neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collision-induced Dissociation
Collision-induced dissociation (CID), also known as collisionally activated dissociation (CAD), is a mass spectrometry technique to induce fragmentation of selected ions in the gas phase. The selected ions (typically molecular ions or protonated molecules) are usually accelerated by applying an electrical potential to increase the ion kinetic energy and then allowed to collide with neutral molecules (often helium, nitrogen or argon). In the collision some of the kinetic energy is converted into internal energy which results in bond breakage and the fragmentation of the molecular ion into smaller fragments. These fragment ions can then be analyzed by tandem mass spectrometry. CID and the fragment ions produced by CID are used for several purposes. Partial or complete structural determination can be achieved. In some cases identity can be established based on previous knowledge without determining structure. Another use is in simply achieving more sensitive and specific detection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium Compounds
Berkelium forms a number of chemical compounds, where it normally exists in an Oxidation number, oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous solution, aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid.Peterson, p. 55Holleman, p. 1956 Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |