|
Berkelium(IV) Oxide
Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C. :2BkO2 + H2 → Bk2O3 + H2O Production Berkelium(IV) oxide is produced by burning berkelium metal in air at 1200 °C. It can also be produced by reacting berkelium(III) oxide with oxygen at 600 °C. References {{Oxides Berkelium compounds Oxides Fluorite crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(IV) Sulfide
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The production of the second-most important isotope, 247Bk, involves the irradiation of the rare isotope 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical appli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Americium(IV) Oxide
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was named after the Americas. Americium was first produced in 1944 by the group of Glenn T. Seaborg from Berkeley, California, at the Metallurgical Laboratory of the University of Chicago, as part of the Manhattan Project. Although it is the third element in the transuranic series, it was discovered fourth, after the heavier curium. The discovery was kept secret and only released to the public in November 1945. Most americium is produced by uranium or plutonium being bombarded with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains about 100 grams of americium. It is widely used in commercial ionization chamber smoke detectors, as well as in neutron sources and industrial gauges. Several unusual applications, such as nucle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
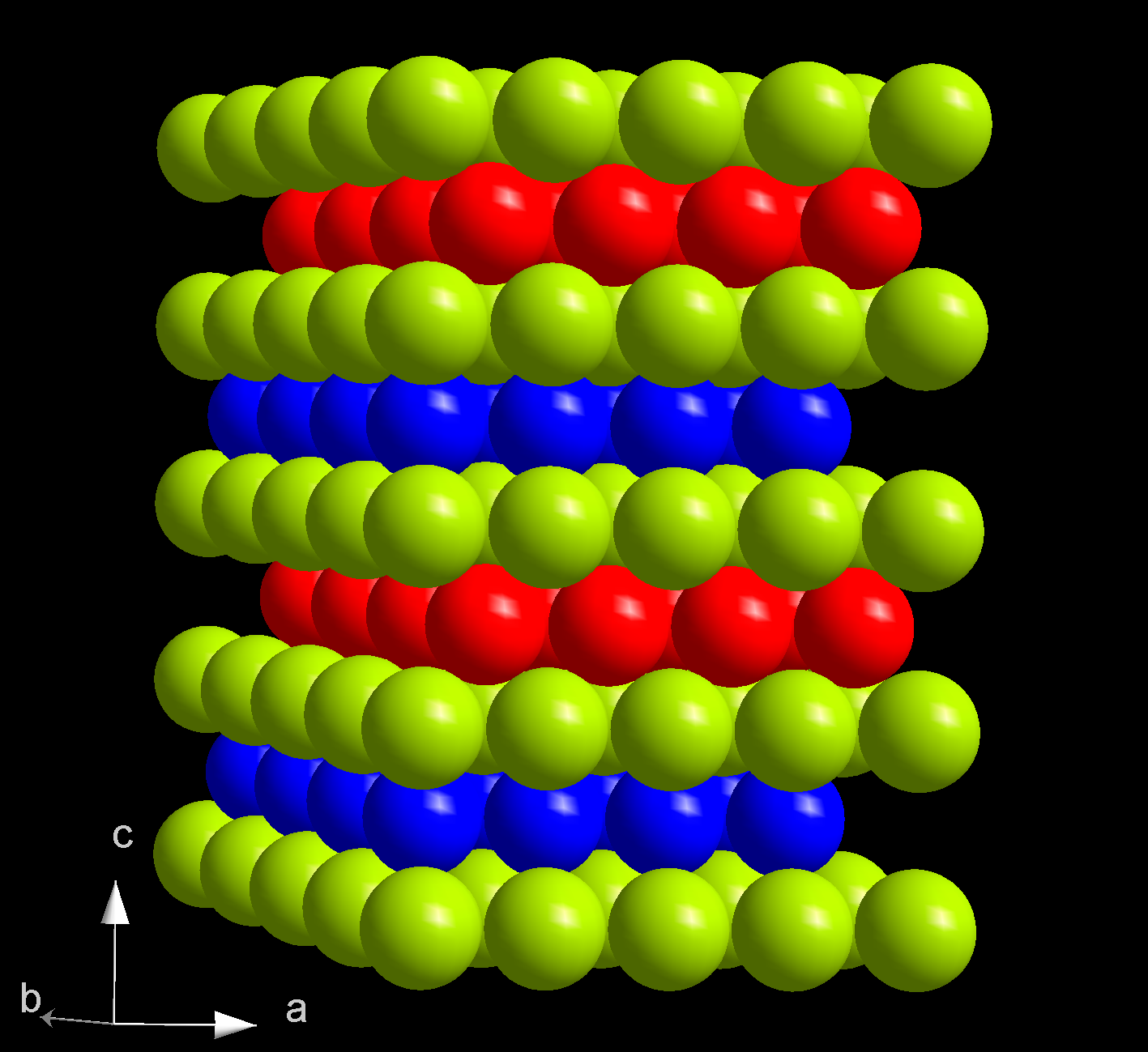
Curium(IV) Oxide
Curium(IV) oxide is an inorganic chemical compound of curium and oxygen with the chemical formula . Since all isotopes of curium are man-made, the compound does not occur in nature. Synthesis *Curium(IV) oxide can be prepared directly from the elements. Metallic curium is annealed in air or in an oxygen atmosphere: :: * Curium(III) hydroxide and curium(III) oxalate are also usually used for this purpose: :: :: *Another way is the reaction of curium(III) oxide in an oxygen atmosphere at 650 °C: :: Physical properties Curium(IV) oxide forms black crystals. Insoluble in water. The compound crystals are of the cubic crystal system In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There ..., the fluorite structure in the space group ''Fm''3''m''. Chemical properties The compound reacts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(IV) Oxide
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding curium with alpha particles (helium-4 ions). It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye (after einsteinium). The element was named after the university and the U.S. state of California. Two crystalline forms exist for californium at normal pressure: one above and one below . A third form exists at high pressure. Californium slowly tarnishes in air at room temperature. Californium compounds are dominated by the +3 oxidation state. The most stable of californium's twenty known isotopes is californium-251, with a half-life of 898 years. This short half-life means the element is not f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(III) Oxide
Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula . Synthesis Berkelium(III) oxide can be prepared from berkelium(IV) oxide by reduction with hydrogen Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...: :: Physical properties The compound forms a yellow-green solid with a melting point of 1920 °C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm. Insoluble in water. References Oxides Berkelium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium(IV) Oxide
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding curium with alpha particles (helium-4 ions). It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye (after einsteinium). The element was named after the university and the U.S. state of California. Two crystalline forms exist for californium at normal pressure: one above and one below . A third form exists at high pressure. Californium slowly tarnishes in air at room temperature. Californium compounds are dominated by the +3 oxidation state. The most stable of californium's twenty known isotopes is californium-251, with a half-life of 898 years. This short half-life means the element is not f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium(III) Oxide
Berkelium(III) oxide is a binary inorganic compound of berkelium and oxygen with the chemical formula . Synthesis Berkelium(III) oxide can be prepared from berkelium(IV) oxide by reduction with hydrogen Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...: :: Physical properties The compound forms a yellow-green solid with a melting point of 1920 °C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm. Insoluble in water. References Oxides Berkelium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The production of the second-most important isotope, 247Bk, involves the irradiation of the rare isotope 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium Compounds
Berkelium forms a number of chemical compounds, where it normally exists in an Oxidation number, oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous solution, aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid.Peterson, p. 55Holleman, p. 1956 Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxides
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |