Berkelium on:
[Wikipedia]
[Google]
[Amazon]
Berkelium is a
 Berkelium is a soft, silvery-white, radioactive
Berkelium is a soft, silvery-white, radioactive

 Although very small amounts of berkelium were possibly produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in December 1949 by
Although very small amounts of berkelium were possibly produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in December 1949 by
/ref> The name choice for element 97 followed the previous tradition of the Californian group to draw an analogy between the newly discovered Further separation was carried out in the presence of a
Further separation was carried out in the presence of a
^_U -> ce^_U -> beta^-23.5 \ \ce] ^_Np -> beta^-2.3565 \ \ce] ^_Pu (the times are ^_Cm ->[][64.15 \ \ce] ^_Bk -> beta^-330 \ \ce] ^_Cf
The thus-produced 249Bk has a long half-life of 330 days and thus can capture another neutron. However, the product, 250Bk, again has a relatively short half-life of 3.212 hours and thus does not yield any heavier berkelium isotopes. It instead decays to the californium isotope 250Cf:
:^_Bk -> ce^_Bk -> beta^-3.212 \ \ce] ^_Cf
Although 247Bk is the most stable isotope of berkelium, its production in nuclear reactors is very difficult because its potential progenitor 247Cm has never been observed to undergo beta decay. Thus, 249Bk is the most accessible isotope of berkelium, which still is available only in small quantities (only 0.66 grams have been produced in the US over the period 1967–1983) at a high price of the order 185 United States dollar, USD per microgram.Hammond C. R. "The elements" in It is the only berkelium isotope available in bulk quantities, and thus the only berkelium isotope whose properties can be extensively studied.
The isotope 248Bk was first obtained in 1956 by bombarding a mixture of curium isotopes with 25 MeV α-particles. Although its direct detection was hindered by strong signal interference with 245Bk, the existence of a new isotope was proven by the growth of the decay product 248Cf which had been previously characterized. The half-life of 248Bk was estimated as hours, though later 1965 work gave a half-life in excess of 300 years (which may be due to an isomeric state). Berkelium-247 was produced during the same year by irradiating 244Cm with alpha-particles:
:
Berkelium-242 was synthesized in 1979 by bombarding 235U with 11B, 238U with 10B, 232Th with 14N or 232Th with 15N. It converts by
 There is currently no use for any isotope of berkelium outside basic scientific research. Berkelium-249 is a common target nuclide to prepare still heavier
There is currently no use for any isotope of berkelium outside basic scientific research. Berkelium-249 is a common target nuclide to prepare still heavier
"Evaluation of nuclear criticality safety. data and limits for actinides in transport"
, p. 16 Berkelium-247 can maintain chain reaction both in a thermal-neutron and in a fast-neutron reactor, however, its production is rather complex and thus the availability is much lower than its critical mass, which is about 75.7 kg for a bare sphere, 41.2 kg with a water reflector and 35.2 kg with a steel reflector (30 cm thickness).
Berkelium
at ''
transuranic
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Bk and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
97. It is a member of the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
and transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
series. It is named after the city of Berkeley, California
Berkeley ( ) is a city on the eastern shore of San Francisco Bay in northern Alameda County, California, United States. It is named after the 18th-century Irish bishop and philosopher George Berkeley. It borders the cities of Oakland and Emer ...
, the location of the Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States Department of Energy National Labs, United States national laboratory that is owned by, and conducts scientific research on behalf of, t ...
(then the University of California
The University of California (UC) is a public land-grant research university system in the U.S. state of California. The system is composed of the campuses at Berkeley, Davis, Irvine, Los Angeles, Merced, Riverside, San Diego, San Francisco, ...
Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium
Neptunium is a chemical element with the Symbol (chemistry), symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after ...
, plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
, curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
and americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
.
The major isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s, mainly at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
in Tennessee
Tennessee ( , ), officially the State of Tennessee, is a landlocked state in the Southeastern region of the United States. Tennessee is the 36th-largest by area and the 15th-most populous of the 50 states. It is bordered by Kentucky to th ...
, United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia
Dimitrovgrad (russian: Димитровград; ), formerly Melekess () until 1972, is a city in Ulyanovsk Oblast, Russia. It is the administrative center of Melekessky District, although it is not within the district and is an independent cit ...
. The production of the second-most important isotope, 247Bk, involves the irradiation of the rare isotope 244Cm with high-energy alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
s.
Just over one gram of berkelium has been produced in the United States since 1967. There is no practical application of berkelium outside scientific research which is mostly directed at the synthesis of heavier transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s and superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s. A 22-milligram batch of berkelium-249 was prepared during a 250-day irradiation period and then purified for a further 90 days at Oak Ridge in 2009. This sample was used to synthesize the new element tennessine
Tennessine is a synthetic chemical element with the symbol Ts and atomic number 117. It is the second-heaviest known element and the penultimate element of the 7th period of the periodic table.
The discovery of tennessine was officially ann ...
for the first time in 2009 at the Joint Institute for Nuclear Research
The Joint Institute for Nuclear Research (JINR, russian: Объединённый институт ядерных исследований, ОИЯИ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research cen ...
, Russia
Russia (, , ), or the Russian Federation, is a List of transcontinental countries, transcontinental country spanning Eastern Europe and North Asia, Northern Asia. It is the List of countries and dependencies by area, largest country in the ...
, after it was bombarded with calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so ...
ions for 150 days. This was the culmination of the Russia–US collaboration on the synthesis of the heaviest elements on the periodic table.
Berkelium is a soft, silvery-white, radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
metal. The berkelium-249 isotope emits low-energy electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s and thus is relatively safe to handle. It decays with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 330 days to californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
-249, which is a strong emitter of ionizing alpha particles. This gradual transformation is an important consideration when studying the properties of elemental berkelium and its chemical compounds, since the formation of californium brings not only chemical contamination, but also free-radical effects and self-heating from the emitted alpha particles.
Characteristics
Physical
 Berkelium is a soft, silvery-white, radioactive
Berkelium is a soft, silvery-white, radioactive actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
metal. In the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, it is located to the right of the actinide curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
, to the left of the actinide californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
and below the lanthanide terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with wa ...
with which it shares many similarities in physical and chemical properties. Its density of 14.78 g/cm3 lies between those of curium (13.52 g/cm3) and californium (15.1 g/cm3), as does its melting point of 986 °C, below that of curium (1340 °C) but higher than that of californium (900 °C). Berkelium is relatively soft and has one of the lowest bulk moduli among the actinides, at about 20 GPa
Grading in education is the process of applying standardized measurements for varying levels of achievements in a course. Grades can be assigned as letters (usually A through F), as a range (for example, 1 to 6), as a percentage, or as a numbe ...
(2 Pa).
ions shows two sharp fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
peaks at 652 nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re ...
s (red light) and 742 nanometers (deep red – near-infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of Light, visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from ...
) due to internal transitions at the f-electron shell. The relative intensity of these peaks depends on the excitation power and temperature of the sample. This emission can be observed, for example, after dispersing berkelium ions in a silicate glass, by melting the glass in presence of berkelium oxide or halide.
Between 70 K and room temperature, berkelium behaves as a Curie–Weiss paramagnetic material with an effective magnetic moment of 9.69 Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\mathrm ...
s (µB) and a Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
of 101 K. This magnetic moment is almost equal to the theoretical value of 9.72 µB calculated within the simple atomic L-S coupling model. Upon cooling to about 34 K, berkelium undergoes a transition to an antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
state. Enthalpy of dissolution
In thermochemistry, the enthalpy of solution ( heat of solution or enthalpy of solvation) is the enthalpy change associated with the dissolution of a substance in a solvent at constant pressure resulting in infinite dilution.
The enthalpy of so ...
in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
at standard conditions is −600 kJ/mol, from which the standard enthalpy of formation (Δf''H''°) of aqueous ions is obtained as −601 kJ/mol. The standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
/Bk is −2.01 V. The ionization potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
of a neutral berkelium atom is 6.23 eV.
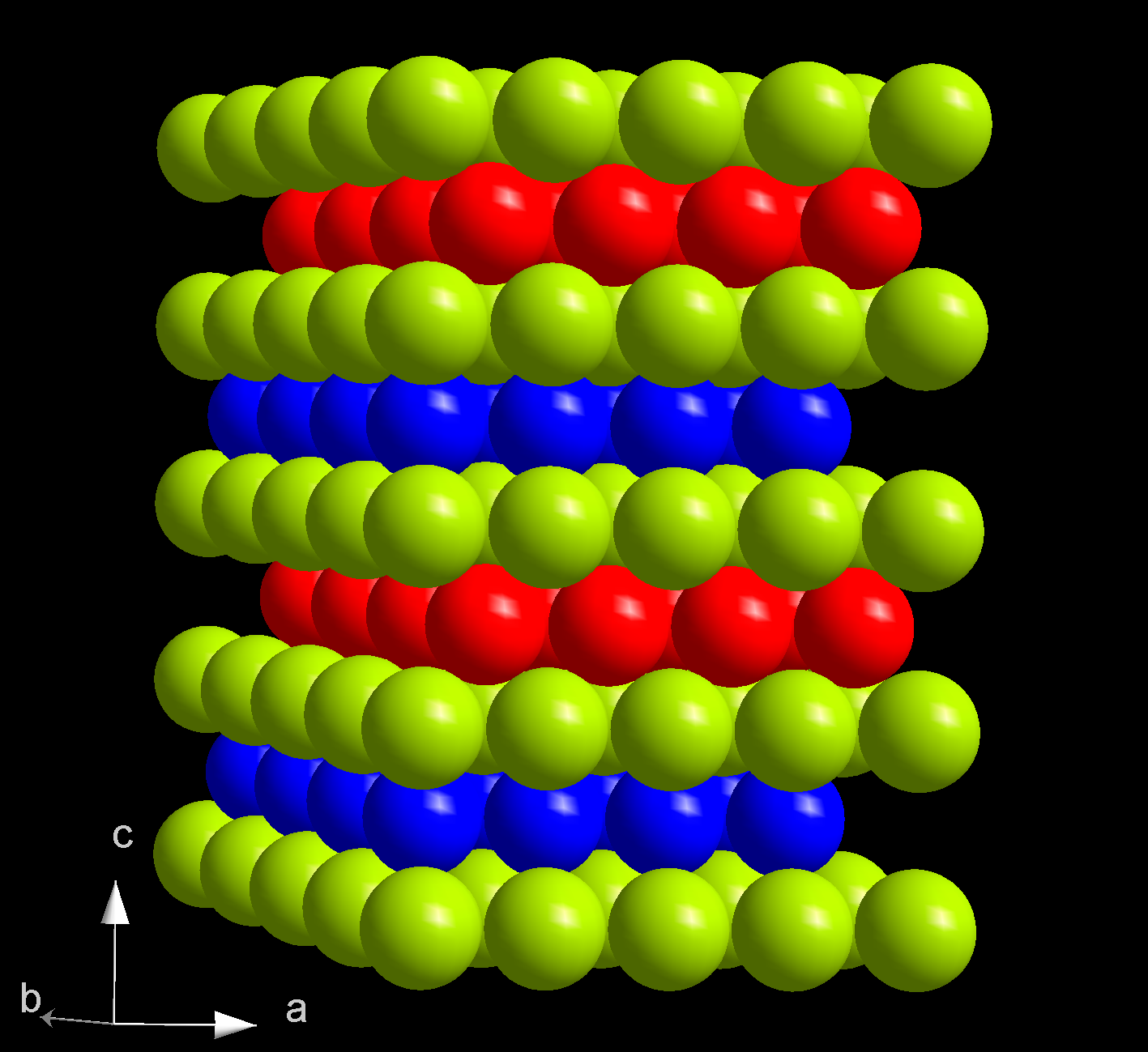
Allotropes
At ambient conditions, berkelium assumes its most stable α form which has ahexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
symmetry, space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
''P63/mmc'', lattice parameters of 341 pm and 1107 pm. The crystal has a double-hexagonal close packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
structure with the layer sequence ABAC and so is isotypic Isostructural chemical compounds have similar chemical structures. " Isomorphous" when used in the relation to crystal structures is not synonymous: in addition to the same atomic connectivity that characterises isostructural compounds, isomorphous ...
(having a similar structure) with α-lanthanum and α-forms of actinides beyond curium. This crystal structure changes with pressure and temperature. When compressed at room temperature to 7 GPa, α-berkelium transforms to the β modification, which has a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
(''fcc'') symmetry and space group ''Fmm''. This transition occurs without change in volume, but the enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
increases by 3.66 kJ/mol. Upon further compression to 25 GPa, berkelium transforms to an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
γ-berkelium structure similar to that of α-uranium. This transition is accompanied by a 12% volume decrease and delocalization of the electrons at the 5f electron shell. No further phase transitions are observed up to 57 GPa.
Upon heating, α-berkelium transforms into another phase with an ''fcc'' lattice (but slightly different from β-berkelium), space group ''Fmm'' and the lattice constant of 500 pm; this ''fcc'' structure is equivalent to the closest packing with the sequence ABC. This phase is metastable and will gradually revert to the original α-berkelium phase at room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. The temperature of the phase transition is believed to be quite close to the melting point.
Chemical
Like allactinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
s, berkelium dissolves in various aqueous inorganic acids, liberating gaseous hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and converting into the state. This trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
(+3) is the most stable, especially in aqueous solutions, but tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
(+4), pentavalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of a ...
(+5), and possibly divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
(+2) berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum(III) chloride-strontium chloride
Strontium chloride (SrCl2) is a salt of strontium and chlorine.
It is a 'typical' salt, forming neutral aqueous solutions. As with all compounds of strontium, this salt emits a bright red colour in flame, and is commonly used in fireworks to that ...
melts. A similar behavior is observed for the lanthanide analogue of berkelium, terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with wa ...
. Aqueous solutions of ions are green in most acids. The color of ions is yellow in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and orange-yellow in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
. Berkelium does not react rapidly with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
at room temperature, possibly due to the formation of a protective oxide layer surface. However, it reacts with molten metals, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
, halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s, chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioact ...
s and pnictogen
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the ele ...
s to form various binary compounds.
Isotopes
About twenty isotopes and sixnuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state, higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited ...
s (excited states of an isotope) of berkelium have been characterized with the mass numbers ranging from 233 to 253 (except 235, 237, and 239). All of them are radioactive. The longest half-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
are observed for 247Bk (1,380 years), 248Bk (over 300 years), and 249Bk (330 days); the half-lives of the other isotopes range from microseconds to several days. The isotope which is the easiest to synthesize is berkelium-249. This emits mostly soft β-particles which are inconvenient for detection. Its alpha radiation
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atom ...
is rather weak (1.45%) with respect to the β-radiation, but is sometimes used to detect this isotope. The second important berkelium isotope, berkelium-247, is an alpha-emitter, as are most actinide isotopes.
Occurrence
All berkelium isotopes have a half-life far too short to be primordial. Therefore, any primordial berkelium − that is, berkelium present on the Earth during its formation − has decayed by now. On Earth, berkelium is mostly concentrated in certain areas, which were used for the atmosphericnuclear weapons tests
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected b ...
between 1945 and 1980, as well as at the sites of nuclear incidents, such as the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
, Three Mile Island accident
The Three Mile Island accident was a partial meltdown of the Three Mile Island, Unit 2 (TMI-2) reactor in Pennsylvania, United States. It began at 4 a.m. on March 28, 1979. It is the most significant accident in U.S. commercial nuclea ...
and 1968 Thule Air Base B-52 crash
On 21 January 1968, an aircraft accident, sometimes known as the Thule affair or Thule accident (; da, Thuleulykken), involving a United States Air Force (USAF) B-52 bomber occurred near Thule Air Base in the Danish territory of Greenland. The ...
. Analysis of the debris at the testing site of the first United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
' first thermonuclear weapon
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
, Ivy Mike
Ivy Mike was the codename given to the first full-scale test of a thermonuclear device, in which part of the explosive yield comes from nuclear fusion.
Ivy Mike was detonated on November 1, 1952, by the United States on the island of Elugelab in ...
, (1 November 1952, Enewetak Atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
), revealed high concentrations of various actinides, including berkelium. For reasons of military secrecy, this result was not published until 1956.
Nuclear reactors produce mostly, among the berkelium isotopes, berkelium-249. During the storage and before the fuel disposal, most of it beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s to californium-249. The latter has a half-life of 351 years, which is relatively long when compared to the other isotopes produced in the reactor, and is therefore undesirable in the disposal products.
The transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s from americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
to fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
, including berkelium, occurred naturally in the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. Th ...
at Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a French ...
, but no longer do so.
Berkelium is also one of the elements that have been detected in Przybylski's Star.
History

 Although very small amounts of berkelium were possibly produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in December 1949 by
Although very small amounts of berkelium were possibly produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in December 1949 by Glenn T. Seaborg
Glenn Theodore Seaborg (; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
, Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
, Stanley Gerald Thompson, and Kenneth Street Jr. They used the 60-inch cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Janu ...
at the University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant u ...
. Similar to the nearly simultaneous discovery of americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
(element 95) and curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
(element 96) in 1944, the new elements berkelium and californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
(element 98) were both produced in 1949–1950.Abstract/ref> The name choice for element 97 followed the previous tradition of the Californian group to draw an analogy between the newly discovered
actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
and the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
element positioned above it in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. Previously, americium was named after a continent as its analogue europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
, and curium honored scientists Marie
Marie may refer to:
People Name
* Marie (given name)
* Marie (Japanese given name)
* Marie (murder victim), girl who was killed in Florida after being pushed in front of a moving vehicle in 1973
* Marie (died 1759), an enslaved Cree person in Tr ...
and Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becqu ...
as the lanthanide above it, gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
, was named after the explorer of the rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
s Johan Gadolin
Johan Gadolin (5 June 176015 August 1852) was a Finnish chemist, physicist and mineralogist. Gadolin discovered a " new earth" containing the first rare-earth compound yttrium, which was later determined to be a chemical element. He is also con ...
. Thus the discovery report by the Berkeley group reads: "It is suggested that element 97 be given the name berkelium (symbol Bk) after the city of Berkeley in a manner similar to that used in naming its chemical homologue terbium
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that is malleable, and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with wa ...
(atomic number 65) whose name was derived from the town of Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is perh ...
, Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
, where the rare earth minerals were first found." This tradition ended with berkelium, though, as the naming of the next discovered actinide, californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, was not related to its lanthanide analogue dysprosium
Dysprosium is the chemical element with the symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it ...
, but after the discovery place.
The most difficult steps in the synthesis of berkelium were its separation from the final products and the production of sufficient quantities of americium for the target material. First, americium ( 241Am) nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
solution was coated on a platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
foil, the solution was evaporated and the residue converted by annealing to americium dioxide
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
(). This target was irradiated with 35 MeV alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
s for 6 hours in the 60-inch cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley. The (α,2n) reaction induced by the irradiation yielded the 243Bk isotope and two free neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s:
: + → + 2
After the irradiation, the coating was dissolved with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
and then precipitated as the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
using concentrated aqueous ammonia solution
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although ...
. The product was centrifugated and re-dissolved in nitric acid. To separate berkelium from the unreacted americium, this solution was added to a mixture of ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
and ammonium sulfate
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen a ...
and heated to convert all the dissolved americium into the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
+6. Unoxidized residual americium was precipitated by the addition of hydrofluoric acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include th ...
as americium(III) fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
(). This step yielded a mixture of the accompanying product curium and the expected element 97 in form of trifluorides. The mixture was converted to the corresponding hydroxides by treating it with potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
, and after centrifugation, was dissolved in perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous sol ...
.
 Further separation was carried out in the presence of a
Further separation was carried out in the presence of a citric acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ...
/ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
buffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
in a weakly acidic medium ( pH≈3.5), using ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
at elevated temperature. The chromatographic
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
separation behavior was unknown for the element 97 at the time, but was anticipated by analogy with terbium. The first results were disappointing because no alpha-particle emission signature could be detected from the elution product. With further analysis, searching for characteristic X-rays and conversion electron
Internal conversion is a non-radioactive, atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal ...
signals, a berkelium isotope was eventually detected. Its mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
was uncertain between 243 and 244 in the initial report, but was later established as 243.
Synthesis and extraction
Preparation of isotopes
Berkelium is produced by bombarding lighter actinidesuranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
(238U) or plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
(239Pu) with neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s in a nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
. In a more common case of uranium fuel, plutonium is produced first by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
(the so-called (n,γ) reaction or neutron fusion) followed by beta-decay:
:half-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
)
Plutonium-239 is further irradiated by a source that has a high neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling ...
, several times higher than a conventional nuclear reactor, such as the 85-megawatt High Flux Isotope Reactor
The High Flux Isotope Reactor (HFIR) is a nuclear research reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, United States. Operating at 85 MW, HFIR is one of the highest flux reactor-based sources of neutrons for condense ...
(HFIR) at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
in Tennessee, USA. The higher flux promotes fusion reactions involving not one but several neutrons, converting 239Pu to 244Cm and then to 249Cm:
:
Curium-249 has a short half-life of 64 minutes, and thus its further conversion to 250Cm has a low probability. Instead, it transforms by beta-decay into 249Bk:
:electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
to 242Cm with a half-life of minutes. A search for an initially suspected isotope 241Bk was then unsuccessful; 241Bk has since been synthesized.
:
Separation
The fact that berkelium readily assumesoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
+4 in solids, and is relatively stable in this state in liquids greatly assists separation of berkelium away from many other actinides. These are inevitably produced in relatively large amounts during the nuclear synthesis and often favor the +3 state. This fact was not yet known in the initial experiments, which used a more complex separation procedure. Various inorganic oxidation agents can be applied to the solutions to convert it to the +4 state, such as bromate
The bromate anion, BrO, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, (), and potassium bromate, ().
Bromates are formed many different ways in municipal drinki ...
s (), bismuthate Bismuthate is an ion. Its chemical formula is BiO3−. It has bismuth in its +5 oxidation state.
It is a very strong oxidizing agent. It reacts with hot water to make bismuth(III) oxide and oxygen. It also reacts with acids. Sodium bismuthate is t ...
s (), chromates ( and ), silver(I) thiolate (), lead(IV) oxide (), ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
(), or photochemical oxidation procedures. More recently, it has been discovered that some organic and bio-inspired molecules, such as the chelator called 3,4,3-LI(1,2-HOPO), can also oxidize Bk(III) and stabilize Bk(IV) under mild conditions. is then extracted with ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
, extraction chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
or liquid-liquid extraction using HDEHP (bis-(2-ethylhexyl) phosphoric acid), amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, tributyl phosphate
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric ac ...
or various other reagents. These procedures separate berkelium from most trivalent actinides and lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
s, except for the lanthanide cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 o ...
(lanthanides are absent in the irradiation target but are created in various nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
decay chains).
A more detailed procedure adopted at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
was as follows: the initial mixture of actinides is processed with ion exchange using lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlor ...
reagent, then precipitated as hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
s, filtered and dissolved in nitric acid. It is then treated with high-pressure elution
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid chromatography experiment, for exa ...
from cation exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
resins, and the berkelium phase is oxidized and extracted using one of the procedures described above. Reduction of the thus-obtained to the +3 oxidation state yields a solution, which is nearly free from other actinides (but contains cerium). Berkelium and cerium are then separated with another round of ion-exchange treatment.
Bulk metal preparation
In order to characterize chemical and physical properties of solid berkelium and its compounds, a program was initiated in 1952 at the Material Testing Reactor,Arco, Idaho
Arco is a city in Butte County, Idaho, United States. The population was 879 as of the 2020 United States census, down from 995 at the 2010 census. Arco is the county seat and largest city in Butte County.
History
Originally known as Root Ho ...
, US. It resulted in preparation of an eight-gram plutonium-239 target and in the first production of macroscopic quantities (0.6 micrograms) of berkelium by Burris B. Cunningham and Stanley Gerald Thompson in 1958, after a continuous reactor irradiation of this target for six years. This irradiation method was and still is the only way of producing weighable amounts of the element, and most solid-state studies of berkelium have been conducted on microgram or submicrogram-sized samples.
The world's major irradiation sources are the 85-megawatt High Flux Isotope Reactor at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
in Tennessee, USA, and the SM-2 loop reactor at the Research Institute of Atomic Reactors (NIIAR) in Dimitrovgrad, Russia
Dimitrovgrad (russian: Димитровград; ), formerly Melekess () until 1972, is a city in Ulyanovsk Oblast, Russia. It is the administrative center of Melekessky District, although it is not within the district and is an independent cit ...
, which are both dedicated to the production of transcurium elements (atomic number greater than 96). These facilities have similar power and flux levels, and are expected to have comparable production capacities for transcurium elements, although the quantities produced at NIIAR are not publicly reported. In a "typical processing campaign" at Oak Ridge, tens of grams of curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
are irradiated to produce decigram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe. ...
quantities of californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, milligram
The kilogram (also kilogramme) is the unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially. ...
quantities of berkelium-249 and einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a compo ...
, and picogram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe. ...
quantities of fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
. In total, just over one gram of berkelium-249 has been produced at Oak Ridge since 1967.
The first berkelium metal sample weighing 1.7 micrograms was prepared in 1971 by the reduction of fluoride with lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
vapor at 1000 °C; the fluoride was suspended on a tungsten wire above a tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is ...
crucible containing molten lithium. Later, metal samples weighing up to 0.5 milligrams were obtained with this method.
:
Similar results are obtained with fluoride. Berkelium metal can also be produced by the reduction of oxide with thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
or lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lantha ...
.
Compounds
Oxides
Two oxides of berkelium are known, with the berkeliumoxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
state of +3 () and +4 ( ). oxide is a brown solid, while oxide is a yellow-green solid with a melting point of 1920 °C and is formed from BkO2 by reduction with molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
:
:
Upon heating to 1200 °C, the oxide undergoes a phase change; it undergoes another phase change at 1750 °C. Such three-phase behavior is typical for the actinide sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides.
Many sesquioxid ...
s. oxide, BkO, has been reported as a brittle gray solid but its exact chemical composition remains uncertain.
Halides
Inhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s, berkelium assumes the oxidation states +3 and +4. The +3 state is the most stable, especially in solutions, while the tetravalent halides and are only known in the solid phase. The coordination of berkelium atom in its trivalent fluoride and chloride is tricapped trigonal prismatic, with the coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
of 9. In trivalent bromide, it is bicapped trigonal prismatic (coordination 8) or octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
(coordination 6), and in the iodide it is octahedral.
fluoride () is a yellow-green ionic solid and is isotypic with uranium tetrafluoride
Uranium tetrafluoride is the inorganic compound with the formula UF4. It is a green solid with an insignificant vapor pressure and low solubility in water. Uranium in its tetravalent (uranous) state is important in various technological processe ...
or zirconium tetrafluoride
Zirconium(IV) fluoride (Zirconium, ZrFluorine, F4) is an inorganic chemical compound. It is a component of ZBLAN fluoride glass. It is insoluble in water. It is the main component of fluorozirconate glasses.
Three crystalline phases of ZrF4 have ...
. fluoride () is also a yellow-green solid, but it has two crystalline structures. The most stable phase at low temperatures is isotypic with yttrium(III) fluoride
Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY( ...
, while upon heating to between 350 and 600 °C, it transforms to the structure found in lanthanum trifluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine.
The LaF3 structure
Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at ...
.
Visible amounts of chloride () were first isolated and characterized in 1962, and weighed only 3 billionths of a gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram.
Originally defined as of 1795 as "the absolute weight of a volume of pure wate ...
. It can be prepared by introducing hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
vapors into an evacuated quartz tube containing berkelium oxide at a temperature about 500 °C. This green solid has a melting point of 600 °C, and is isotypic with uranium(III) chloride
Uranium(III) chloride, UCl3, is a water soluble salt of uranium. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized in various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
Prepar ...
. Upon heating to nearly melting point, converts into an orthorhombic phase.
Two forms of bromide are known: one with berkelium having coordination 6, and one with coordination 8. The latter is less stable and transforms to the former phase upon heating to about 350 °C. An important phenomenon for radioactive solids has been studied on these two crystal forms: the structure of fresh and aged 249BkBr3 samples was probed by X-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
over a period longer than 3 years, so that various fractions of berkelium-249 had beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
ed to californium-249. No change in structure was observed upon the 249BkBr3—249CfBr3 transformation. However, other differences were noted for 249BkBr3 and 249CfBr3. For example, the latter could be reduced with hydrogen to 249CfBr2, but the former could not – this result was reproduced on individual 249BkBr3 and 249CfBr3 samples, as well on the samples containing both bromides. The intergrowth of californium in berkelium occurs at a rate of 0.22% per day and is an intrinsic obstacle in studying berkelium properties. Beside a chemical contamination, 249Cf, being an alpha emitter, brings undesirable self-damage of the crystal lattice and the resulting self-heating. The chemical effect however can be avoided by performing measurements as a function of time and extrapolating the obtained results.
Other inorganic compounds
Thepnictides
A pnictogen ( or ; from grc, wikt:πνίγω, πνῑ́γω "to choke" and wikt:-gen#English, -gen, "generator") is any of the chemical elements in group (periodic table), group 15 of the periodic table. Group 15 is also known as the nitro ...
of berkelium-249 of the type BkX are known for the elements nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient time ...
. They crystallize in the rock-salt structure
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pi ...
and are prepared by the reaction of either hydride () or metallic berkelium with these elements at elevated temperature (about 600 °C) under high vacuum.
sulfide, , is prepared by either treating berkelium oxide with a mixture of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non ...
vapors at 1130 °C, or by directly reacting metallic berkelium with elemental sulfur. These procedures yield brownish-black crystals.
and hydroxides are both stable in 1 molar solutions of sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
. phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
() has been prepared as a solid, which shows strong fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
under excitation with a green light. Berkelium hydrides are produced by reacting metal with hydrogen gas at temperatures about 250 °C. They are non-stoichiometric with the nominal formula (0 < ''x'' < 1). Several other salts of berkelium are known, including an oxysulfide (), and hydrated nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
(), chloride (), sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
() and oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl o ...
(). Thermal decomposition at about 600 °C in an argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
atmosphere (to avoid oxidation to ) of yields the crystals of oxysulfate (). This compound is thermally stable to at least 1000 °C in inert atmosphere.
Organoberkelium compounds
Berkelium forms a trigonal (η5–C5H5)3Bkmetallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
complex with three cyclopentadienyl Cyclopentadienyl can refer to
*Cyclopentadienyl anion, or cyclopentadienide,
**Cyclopentadienyl ligand
*Cyclopentadienyl radical, •
*Cyclopentadienyl cation,
See also
*Pentadienyl
In organic chemistry, pentadienyl refers to the organic radic ...
rings, which can be synthesized by reacting chloride with the molten beryllocene () at about 70 °C. It has an amber color and a density of 2.47 g/cm3. The complex is stable to heating to at least 250 °C, and sublimates without melting at about 350 °C. The high radioactivity of berkelium gradually destroys the compound (within a period of weeks). One cyclopentadienyl ring in (η5–C5H5)3Bk can be substituted by chlorine to yield . The optical absorption spectra of this compound are very similar to those of (η5–C5H5)3Bk.
Applications
 There is currently no use for any isotope of berkelium outside basic scientific research. Berkelium-249 is a common target nuclide to prepare still heavier
There is currently no use for any isotope of berkelium outside basic scientific research. Berkelium-249 is a common target nuclide to prepare still heavier transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s and superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s,Stwertka, Albert. ''A Guide to the Elements'', Oxford University Press, 1996, p. 211. such as lawrencium
Lawrencium is a synthetic chemical element with the symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radi ...
, rutherfordium
Rutherfordium is a chemical element with the symbol Rf and atomic number 104, named after New Zealand-born British physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a laboratory. It is radioactiv ...
and bohrium
Bohrium is a synthetic chemical element with the symbol Bh and atomic number 107. It is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in a laboratory but is not found in nature. All known isotopes of bohriu ...
. It is also useful as a source of the isotope californium-249, which is used for studies on the chemistry of californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
in preference to the more radioactive californium-252 that is produced in neutron bombardment facilities such as the HFIR.
A 22 milligram batch of berkelium-249 was prepared in a 250-day irradiation and then purified for 90 days at Oak Ridge in 2009. This target yielded the first 6 atoms of tennessine
Tennessine is a synthetic chemical element with the symbol Ts and atomic number 117. It is the second-heaviest known element and the penultimate element of the 7th period of the periodic table.
The discovery of tennessine was officially ann ...
at the Joint Institute for Nuclear Research
The Joint Institute for Nuclear Research (JINR, russian: Объединённый институт ядерных исследований, ОИЯИ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research cen ...
(JINR), Dubna
Dubna ( rus, Дубна́, p=dʊbˈna) is a town in Moscow Oblast, Russia. It has a status of ''naukograd'' (i.e. town of science), being home to the Joint Institute for Nuclear Research, an international nuclear physics research center and one o ...
, Russia, after bombarding it with calcium ions in the U400 cyclotron for 150 days. This synthesis was a culmination of the Russia-US collaboration between JINR and Lawrence Livermore National Laboratory
Lawrence Livermore National Laboratory (LLNL) is a federal research facility in Livermore, California, United States. The lab was originally established as the University of California Radiation Laboratory, Livermore Branch in 1952 in response ...
on the synthesis of elements 113 to 118 which was initiated in 1989.
Nuclear fuel cycle
Thenuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
properties of berkelium are different from those of the neighboring actinides curium and californium, and they suggest berkelium to perform poorly as a fuel in a nuclear reactor. Specifically, berkelium-249 has a moderately large neutron capture cross section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture & engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**Abs ...
of 710 barns
A barn is an agricultural building usually on farms and used for various purposes. In North America, a barn refers to structures that house livestock, including cattle and horses, as well as equipment and fodder, and often grain.Allen G. N ...
for thermal neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
, 1200 barns resonance integral
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
, but very low fission cross section for thermal neutrons. In a thermal reactor, much of it will therefore be converted to berkelium-250 which quickly decays to californium-250. In principle, berkelium-249 can sustain a nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
in a fast breeder reactor
A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. Breeder reactors achieve this because their neutron economy is high enough to create more fissile fuel than they use, by irradiation of a fertile mate ...
. Its critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fissi ...
is relatively high at 192 kg; it can be reduced with a water or steel reflector but would still exceed the world production of this isotope.Institut de Radioprotection et de Sûreté Nucléaire"Evaluation of nuclear criticality safety. data and limits for actinides in transport"
, p. 16 Berkelium-247 can maintain chain reaction both in a thermal-neutron and in a fast-neutron reactor, however, its production is rather complex and thus the availability is much lower than its critical mass, which is about 75.7 kg for a bare sphere, 41.2 kg with a water reflector and 35.2 kg with a steel reflector (30 cm thickness).
Health issues
Little is known about the effects of berkelium on human body, and analogies with other elements may not be drawn because of different radiation products (electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s for berkelium and alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
s, neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s, or both for most other actinides). The low energy of electrons emitted from berkelium-249 (less than 126 keV) hinders its detection, due to signal interference with other decay processes, but also makes this isotope relatively harmless to humans as compared to other actinides. However, berkelium-249 transforms with a half-life of only 330 days to the strong alpha-emitter californium-249, which is rather dangerous and has to be handled in a glovebox
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their han ...
in a dedicated laboratory.
Most available berkelium toxicity data originate from research on animals. Upon ingestion by rats, only about 0.01% of berkelium ends in the blood stream. From there, about 65% goes to the bones, where it remains for about 50 years, 25% to the lungs (biological half-life about 20 years), 0.035% to the testicles or 0.01% to the ovaries where berkelium stays indefinitely. The balance of about 10% is excreted. In all these organs berkelium might promote cancer, and in the skeleton
A skeleton is the structural frame that supports the body of an animal. There are several types of skeletons, including the exoskeleton, which is the stable outer shell of an organism, the endoskeleton, which forms the support structure inside ...
, its radiation can damage red blood cells. The maximum permissible amount of berkelium-249 in the human skeleton is 0.4 nanogram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe. ...
s.Pradyot Patnaik. ''Handbook of Inorganic Chemicals'' McGraw-Hill, 2002,
References
Bibliography
* * *External links
Berkelium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{good article
Chemical elements
Chemical elements with double hexagonal close-packed structure
Actinides
Synthetic elements