|
Chromate And Dichromate
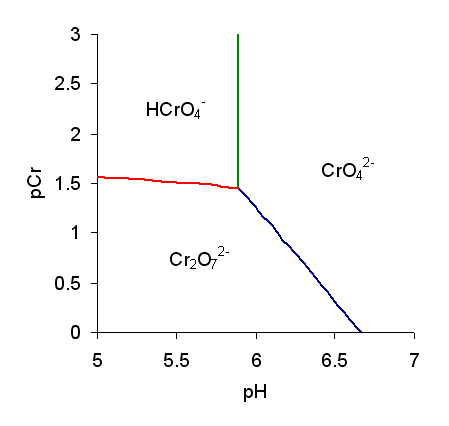
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, potassium chromate Potassium-dichromate-sample.jpg, potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex CrO(O2)2py. Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. :2 + 2 H+ + H2O The predominance diagram shows that the position of the equilibrium depends on b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ball-and-stick Model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the three-dimensional position of the atoms and the bonds between them. The atoms are typically represented by spheres, connected by rods which represent the bonds. Double and triple bonds are usually represented by two or three curved rods, respectively, or alternately by correctly positioned sticks for the sigma and pi bonds. In a good model, the angles between the rods should be the same as the angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nuclei. The chemical element of each atom is often indicated by the sphere's color. In a ball-and-stick model, the radius of the spheres is usually much smaller than the rod lengths, in order to provide a clearer view of the atoms and bonds throughout the model. As a consequence, the model does not provide a clear insight about th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Metals
upright=1.2, Crystals of osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead Heavy metals are generally defined as metals with relatively high density, densities, atomic weights, or atomic numbers. The criteria used, and whether metalloids are included, vary depending on the author and context. In metallurgy, for example, a heavy metal may be defined on the basis of density, whereas in physics the distinguishing criterion might be atomic number, while a chemist would likely be more concerned with chemical property, chemical behaviour. More specific definitions have been published, but none of these have been widely accepted. The definitions surveyed in this article encompass up to 96 out of the 118 known chemical elements; only mercury, lead and bismuth meet all of them. Despite this lack of agreement, the term (plural or singular) is widely used in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chrome Plating
Chrome plating (less commonly chromium plating) is a technique of electroplating a thin layer of chromium onto a metal object. A chrome-plated item is called ''chrome''. The chromed layer can be decorative, provide corrosion resistance, ease of cleaning, or increase surface hardness. Sometimes, a less expensive imitator of chrome may be used for aesthetic purposes. Process Chrome plating a component typically includes these stages: * Degreasing to remove heavy soiling * Manual cleaning to remove all residual traces of dirt and surface impurities * Various pretreatments depending on the substrate * Placement into the chrome plating vat, where it is allowed to warm to solution temperature * Application of plating current for the required time to attain the desired thickness There are many variations to this process, depending on the type of substrate being plated. Different substrates need different etching solutions, such as hydrochloric, hydrofluoric, and sulfuric acids. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexavalent Chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the salt sodium dichromate. Hexavalent chromium is key to all materials made from chromium. Approximately of hexavalent chromium were produced in 1985. Additional hexavalent chromium compounds include chromium trioxide and various salts of chromate and dichromate, among others. Hexavalent chromium is used in textile dyes, wood preservation, anti-corrosion products, chromate conversion coatings, and a variety of niche uses. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anticorrosive agents to paints, primers, and other surface coatings; and chromic acid electroplated onto metal parts to provide a decorative or protective coating. Hexavalent chromium can be fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laidlaw School Bus
Laidlaw (), organized as Laidlaw International, Inc. (with corporate headquarters in Naperville, Illinois) was the largest provider of intercity bus services, contract public transit and paratransit, and contract school bus service in both the United States and Canada. In February 2007, FirstGroup, a bus and rail transportation operator in the United Kingdom with subsidiaries in North America, acquired Laidlaw International, Inc. FirstGroup completed the acquisition of Laidlaw International on October 1, 2007, and rebranded Laidlaw services under the First umbrella. The deal combined North America’s two largest private school bus operators—Education Services and First Student Inc.—giving them a combined 40% of the school bus contractor market. Laidlaw had grown primarily through acquisitions of other companies and contracting of services formerly directly provided by government entities. It was the parent company of Laidlaw Transit (which was merged into First Transit) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential (reduction potential is more often used due to general formalism in electrochemistry), the greater the species' affinity for electrons and tendency to be reduced. Measurement and interpretation In aqueous solutions, redox potential is a measure of the tendency of the solution to either gain or lose electrons when it is subjected to change by introduction of a new species. A solution with a higher (more positive) reduction potential than the new species will have a tendency to gain electrons from the new species (i.e. to be reduced by oxidizing the new species) and a solution with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reduction (chemistry)
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Dissociation Constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction :HA A^- + H^+ known as dissociation in the context of acid–base reactions. The chemical species HA is an acid that dissociates into , the conjugate base of the acid and a hydrogen ion, . The system is said to be in equilibrium when the concentrations of its components will not change over time, because both forward and backward reactions are occurring at the same rate. The dissociation constant is defined by :K_\text = \mathrm, or :\mathrmK_\ce = - \log_ K_\text = \log_\frac where quantities in square brackets represent the concentrations of the species at equilibrium. Theoretical background The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction; the p''K''a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromic Acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, H2CrO4 of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (or VI). It is a strong and corrosive oxidising agent. Molecular chromic acid Molecular chromic acid, H2CrO4, has much in common with sulfuric acid, H2SO4. Only sulfuric acid can be classified as part of the 7 strong acids list. Due to the laws pertinent to the concept of "first order ionization energy", the first proton is lost most easily. It behaves extremely similar to sulfuric acid deprotonation. Since the process of polyvalent acid-base titrations have more than one proton (especially when the acid is starting substance and the base is the titrant), proton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weak Acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a hydron (chemistry), proton, H+, and an anion, A-. The Dissociation (chemistry), dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. :HA -> H+ + A- Examples of strong acids are hydrochloric acid (HCl), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). A weak acid is only partially dissociated, with both the undissociated acid and its dissociation products being present, in solution, in Equilibrium chemistry, equilibrium with each other. :HA H+ + A- Acetic acid (CH3COOH) is an example of a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, K_\ce value. The strength of a weak organic chemistry, organic acid may depend on substituent effects. The strength of an inorganic chemistry, inorganic acid is dependent on the oxidation state for the atom to which the prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



-oxid.jpg)