Carbon Cycle-animated Forest on:
[Wikipedia]
[Google]
[Amazon]
Carbon () is a
 Carbon in its solid state exists in several
Carbon in its solid state exists in several
 Carbon combines with some metals at high temperatures to form metallic carbides, such as the iron carbide
Carbon combines with some metals at high temperatures to form metallic carbides, such as the iron carbide
 The
The  At very high pressures, carbon forms the more compact allotrope, diamond, having nearly twice the density of graphite. Here, each atom is bonded tetrahedrally to four others, forming a 3-dimensional network of puckered six-membered rings of atoms. Diamond has the same cubic structure as
At very high pressures, carbon forms the more compact allotrope, diamond, having nearly twice the density of graphite. Here, each atom is bonded tetrahedrally to four others, forming a 3-dimensional network of puckered six-membered rings of atoms. Diamond has the same cubic structure as

 Carbon is the fourth most abundant chemical element in the observable universe by mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets. Some
Carbon is the fourth most abundant chemical element in the observable universe by mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets. Some
greatly upgraded database
for tracking
 Under terrestrial conditions, conversion of one element to another is very rare. Therefore, the amount of carbon on Earth is effectively constant. Thus, processes that use carbon must obtain it from somewhere and dispose of it somewhere else. The paths of carbon in the environment form the
Under terrestrial conditions, conversion of one element to another is very rare. Therefore, the amount of carbon on Earth is effectively constant. Thus, processes that use carbon must obtain it from somewhere and dispose of it somewhere else. The paths of carbon in the environment form the

 Carbon can form very long chains of interconnecting
Carbon can form very long chains of interconnecting
 The English name ''carbon'' comes from the Latin ''carbo'' for coal and charcoal, whence also comes the French ''charbon'', meaning charcoal. In German, Dutch and Danish, the names for carbon are ''Kohlenstoff'', ''koolstof'', and ''kulstof'' respectively, all literally meaning coal-substance.
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.
The English name ''carbon'' comes from the Latin ''carbo'' for coal and charcoal, whence also comes the French ''charbon'', meaning charcoal. In German, Dutch and Danish, the names for carbon are ''Kohlenstoff'', ''koolstof'', and ''kulstof'' respectively, all literally meaning coal-substance.
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.
 In 1722, René Antoine Ferchault de Réaumur demonstrated that iron was transformed into steel through the absorption of some substance, now known to be carbon. In 1772,
In 1722, René Antoine Ferchault de Réaumur demonstrated that iron was transformed into steel through the absorption of some substance, now known to be carbon. In 1772,
and Graphite: Mineral Commodity Summaries 2011 There are three types of natural graphite—amorphous, flake or crystalline flake, and vein or lump. Amorphous graphite is the lowest quality and most abundant. Contrary to science, in industry "amorphous" refers to very small crystal size rather than complete lack of crystal structure. Amorphous is used for lower value graphite products and is the lowest priced graphite. Large amorphous graphite deposits are found in China, Europe, Mexico and the United States. Flake graphite is less common and of higher quality than amorphous; it occurs as separate plates that crystallized in metamorphic rock. Flake graphite can be four times the price of amorphous. Good quality flakes can be processed into expandable graphite for many uses, such as
 The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of
 Pure carbon has extremely low toxicity to humans and can be handled safely in the form of graphite or charcoal. It is resistant to dissolution or chemical attack, even in the acidic contents of the digestive tract. Consequently, once it enters into the body's tissues it is likely to remain there indefinitely. Carbon black was probably one of the first pigments to be used for tattooing, and
Pure carbon has extremely low toxicity to humans and can be handled safely in the form of graphite or charcoal. It is resistant to dissolution or chemical attack, even in the acidic contents of the digestive tract. Consequently, once it enters into the body's tissues it is likely to remain there indefinitely. Carbon black was probably one of the first pigments to be used for tattooing, and
Carbon
at ''
Carbon on Britannica
(archived 18 June 2010)
(archived 9 November 2001)
Carbon—Super Stuff. Animation with sound and interactive 3D-models.
(archived 9 November 2012) {{Authority control Allotropes of carbon Chemical elements with hexagonal planar structure Chemical elements Native element minerals Polyatomic nonmetals Reactive nonmetals Reducing agents
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
C and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
6. It is nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
lic and tetravalent—meaning that its atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
s are able to form up to four covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s due to its valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
exhibiting 4 electrons. It belongs to group 14 of the periodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
occur naturally, C and C being stable, while C is a radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
, decaying with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 5,700 years. Carbon is one of the few elements known since antiquity.
Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
, and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. Carbon's abundance, its unique diversity of organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s, and its unusual ability to form polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen.
The atoms of carbon can bond together in diverse ways, resulting in various allotropes of carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency (Tetravalence, tetravalent). Well-known forms of carbon include diamond and graphite. In recent ...
. Well-known allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
include graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
, amorphous carbon
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amo ...
, and fullerenes
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- ...
. The physical properties
A physical property is any property of a physical system that is measurable. The changes in the physical properties of a system can be used to describe its changes between momentary states. A quantifiable physical property is called ''physical ...
of carbon vary widely with the allotropic form. For example, graphite is opaque
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shie ...
and black, while diamond is highly transparent. Graphite is soft enough to form a streak on paper (hence its name, from the Greek verb "γράφειν" which means "to write"), while diamond is the hardest naturally occurring material known. Graphite is a good electrical conductor
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. The flow of negatively c ...
while diamond has a low electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
. Under normal conditions, diamond, carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s, and graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
have the highest thermal conductivities of all known materials. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable
In chemistry, chemical stability is the thermodynamic stability of a chemical system, in particular a chemical compound or a polymer. Colloquially, it may instead refer to kinetic persistence, the shelf-life of a metastable substance or system; th ...
form at standard temperature and pressure. They are chemically resistant and require high temperature to react even with oxygen.
The most common oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of carbon in inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
s is +4, while +2 is found in carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
and transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
complexes. The largest sources of inorganic carbon are limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
s, dolomites
The Dolomites ( ), also known as the Dolomite Mountains, Dolomite Alps or Dolomitic Alps, are a mountain range in northeastern Italy. They form part of the Southern Limestone Alps and extend from the River Adige in the west to the Piave Va ...
and carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, but significant quantities occur in organic deposits of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
, peat
Peat is an accumulation of partially Decomposition, decayed vegetation or organic matter. It is unique to natural areas called peatlands, bogs, mires, Moorland, moors, or muskegs. ''Sphagnum'' moss, also called peat moss, is one of the most ...
, oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) and lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturate ...
, and methane clathrate
Methane clathrate (CH4·5.75H2O) or (4CH4·23H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large a ...
s. Carbon forms a vast number of compounds, with about two hundred million having been described and indexed; and yet that number is but a fraction of the number of theoretically possible compounds under standard conditions.
Characteristics
 Carbon in its solid state exists in several
Carbon in its solid state exists in several allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
, including graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, a soft, black, and slippery material, and diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
, the hardest naturally occurring substance. This variation in physical properties arises from differences in atomic arrangement: graphite consists of layers of hexagonally arranged carbon atoms, while diamond features a rigid three-dimensional lattice.
Chemically, carbon is notable for its ability to form stable chemical bonds
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as ...
with many elements, particularly with other carbon atoms, and is capable of forming multiple stable covalent bonds
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
with suitable multivalent atoms. Carbon is a component element in the large majority of all chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s, with about two hundred million examples having been described in the published chemical literature. Carbon also has the highest sublimation point of all elements. At atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
it has no melting point, as its triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at ...
is at and , so it sublimes at about .
Compared to its well-known solid allotropes, the liquid and gaseous phases of carbon are far less studied. In the vapor phase, some of the carbon is in the form of highly reactive diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
dicarbon (). When excited, this gas glows green. The liquid phase of carbon is a dark, mobile, and reflective liquid that can only exist above and under pressures exceeding 100 atmospheres.
Carbon is the sixth element, with a ground-state electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
of 1s22s22p2, of which the four outer electrons are valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s. Its first four ionisation energies, 1086.5, 2352.6, 4620.5 and 6222.7 kJ/mol, are much higher than those of the heavier group-14 elements. The electronegativity of carbon is 2.5, significantly higher than the heavier group-14 elements (1.8–1.9), but close to most of the nearby nonmetals, as well as some of the second- and third-row transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s. Carbon's covalent radii are normally taken as 77.2 pm (C−C), 66.7 pm (C=C) and 60.3 pm (C≡C), although these may vary depending on coordination number and what the carbon is bonded to. In general, covalent radius decreases with lower coordination number and higher bond order.
Chemical
Graphite is much more reactive than diamond at standard conditions, despite being more thermodynamically stable, as its delocalisedpi system
In mathematics, a -system (or pi-system) on a set \Omega is a collection P of certain subsets of \Omega, such that
* P is non-empty.
* If A, B \in P then A \cap B \in P.
That is, P is a non-empty family of subsets of \Omega that is closed und ...
is much more vulnerable to attack. For example, graphite can be oxidised by hot concentrated nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
at standard conditions to mellitic acid, C6(CO2H)6, which preserves the hexagonal units of graphite while breaking up the larger structure.
Carbon-based compounds form the basis of all known life on Earth, and the carbon-nitrogen-oxygen cycle provides a small portion of the energy produced by the Sun, and most of the energy in larger stars (e.g. Sirius
Sirius is the brightest star in the night sky. Its name is derived from the Greek word (Latin script: ), meaning 'glowing' or 'scorching'. The star is designated Canis Majoris, Latinized to Alpha Canis Majoris, and abbr ...
). Although it forms an extraordinary variety of compounds, most forms of carbon are comparatively unreactive under normal conditions. At standard temperature and pressure, it resists all but the strongest oxidizers. It does not react with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
or any alkalis
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
. At elevated temperatures, carbon reacts with oxygen to form carbon oxides and will rob oxygen from metal oxides to leave the elemental metal. This exothermic reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC define ...
is used in the iron and steel industry to smelt iron and to control the carbon content of steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength a ...
:
: + 4 C + 2 → 3 Fe + 4 .
Carbon reacts with sulfur to form carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
, and it reacts with steam in the coal-gas reaction used in coal gasification
In industrial chemistry, coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (), carbon dioxide (), methane (), and water vapour ()—from coal and water, air and/or oxygen.
H ...
:
:C + HO → CO + H.
 Carbon combines with some metals at high temperatures to form metallic carbides, such as the iron carbide
Carbon combines with some metals at high temperatures to form metallic carbides, such as the iron carbide cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, b ...
in steel and tungsten carbide
Tungsten carbide (chemical formula: ) is a carbide containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into shapes through sintering for use in in ...
, widely used as an abrasive and for making hard tips for cutting tools.
Allotropes
Atomic carbon is a very short-lived species and, therefore, carbon is stabilized in various multi-atomic structures with diverse molecular configurations calledallotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
s. The three relatively well-known allotropes of carbon are amorphous carbon
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amo ...
, graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, and diamond. Once considered exotic, fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
s are nowadays commonly synthesized and used in research; they include buckyball
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
s, carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s, carbon nanobud
In nanotechnology, a carbon nanobud is a material that combines carbon nanotubes and spheroidal fullerenes, both allotropes of carbon, forming "buds" attached to the tubes. Carbon nanobuds were discovered and synthesized in 2006.
In this mat ...
s and nanofibers
Nanofibers are fibers with diameters in the Nanometre, nanometer range (typically, between 1 nm and 1 μm). Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples ...
. Several other exotic allotropes have also been discovered, such as lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found ...
, glassy carbon, carbon nanofoam Carbon nanofoam is an allotropes of carbon, allotrope of carbon discovered in 1997 by Andrei V. Rode and co-workers at the Australian National University in Canberra. It consists of a cluster-assembly of carbon atoms strung together in a loose three ...
and linear acetylenic carbon
Linear acetylenic carbon (LAC), also known as carbyne or a Linear Carbon Chain (LCC), is an Allotropes of carbon, allotrope of carbon that has the chemical structure as a repeat unit, with alternating Single bond, single and triple bonds. It w ...
(carbyne).
The system of carbon allotropes spans a range of extremes:
Graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
is a two-dimensional sheet of carbon with the atoms arranged in a hexagonal lattice. As of 2009, graphene appears to be the strongest material ever tested.
* The process of separating it from graphite will require some further technological development before it is economical for industrial processes. If successful, graphene could be used in the construction of a space elevator
A space elevator, also referred to as a space bridge, star ladder, and orbital lift, is a proposed type of planet-to-space transportation system, often depicted in science fiction. The main component would be a cable (also called a tether) an ...
. It could also be used to safely store hydrogen for use in a hydrogen based engine in cars.
 The
The amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
form is an assortment of carbon atoms in a non-crystalline, irregular, glassy state, not held in a crystalline macrostructure. It is present as a powder, and is the main constituent of substances such as charcoal, lampblack
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid catalyt ...
(soot), and activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that greatly increase the surface ar ...
. At normal pressures, carbon takes the form of graphite, in which each atom is bonded trigonally to three others in a plane composed of fused hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A regular hexagon is de ...
al rings, just like those in aromatic hydrocarbon
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
s. The resulting network is 2-dimensional, and the resulting flat sheets are stacked and loosely bonded through weak van der Waals force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical elec ...
s. This gives graphite its softness and its cleaving properties (the sheets slip easily past one another). Because of the delocalization of one of the outer electrons of each atom to form a π-cloud, graphite conducts electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by Maxwel ...
, but only in the plane of each covalently bonded
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
sheet. This results in a lower bulk electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
for carbon than for most metals. The delocalization also accounts for the energetic stability of graphite over diamond at room temperature.
silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and germanium
Germanium is a chemical element; it has Symbol (chemistry), symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid or a nonmetal in the carbon group that is chemically ...
, and because of the strength of the carbon-carbon bonds, it is the hardest naturally occurring substance measured by resistance to scratching. Contrary to the popular belief that ''"diamonds are forever"'', they are thermodynamically unstable ( Δf''G''°(diamond, 298 K) = 2.9 kJ/mol) under normal conditions (298 K, 105 Pa) and should theoretically transform into graphite. But due to a high activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
barrier, the transition into graphite is so slow at normal temperature that it is unnoticeable. However, at very high temperatures diamond will turn into graphite, and diamonds can burn up in a house fire. The bottom left corner of the phase diagram for carbon has not been scrutinized experimentally. Although a computational study employing density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
methods reached the conclusion that as and , diamond becomes more stable than graphite by approximately 1.1 kJ/mol, more recent and definitive experimental and computational studies show that graphite is more stable than diamond for , without applied pressure, by 2.7 kJ/mol at ''T'' = 0 K and 3.2 kJ/mol at ''T'' = 298.15 K. Under some conditions, carbon crystallizes as lonsdaleite
Lonsdaleite (named in honour of Kathleen Lonsdale), also called hexagonal diamond in reference to the crystal structure, is an allotrope of carbon with a hexagonal lattice, as opposed to the cubical lattice of conventional diamond. It is found ...
, a hexagonal crystal lattice with all atoms covalently bonded and properties similar to those of diamond.
Fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
s are a synthetic crystalline formation with a graphite-like structure, but in place of flat hexagonal cells only, some of the cells of which fullerenes are formed may be pentagons, nonplanar hexagons, or even heptagons of carbon atoms. The sheets are thus warped into spheres, ellipses, or cylinders. The properties of fullerenes (split into buckyballs, buckytubes, and nanobuds) have not yet been fully analyzed and represent an intense area of research in nanomaterial
Nanomaterials describe, in principle, chemical substances or materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science ...
s. The names ''fullerene'' and ''buckyball'' are given after Richard Buckminster Fuller
Richard Buckminster Fuller (; July 12, 1895 – July 1, 1983) was an American architect, systems theorist, writer, designer, inventor, philosopher, and futurist. He styled his name as R. Buckminster Fuller in his writings, publishing more th ...
, popularizer of geodesic dome
A geodesic dome is a hemispherical thin-shell structure (lattice-shell) based on a geodesic polyhedron. The rigid triangular elements of the dome distribute stress throughout the structure, making geodesic domes able to withstand very heavy ...
s, which resemble the structure of fullerenes. The buckyballs are fairly large molecules formed completely of carbon bonded trigonally, forming spheroid
A spheroid, also known as an ellipsoid of revolution or rotational ellipsoid, is a quadric surface (mathematics), surface obtained by Surface of revolution, rotating an ellipse about one of its principal axes; in other words, an ellipsoid with t ...
s (the best-known and simplest is the soccerball-shaped C buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
). Carbon nanotubes (buckytubes) are structurally similar to buckyballs, except that each atom is bonded trigonally in a curved sheet that forms a hollow cylinder
A cylinder () has traditionally been a three-dimensional solid, one of the most basic of curvilinear geometric shapes. In elementary geometry, it is considered a prism with a circle as its base.
A cylinder may also be defined as an infinite ...
. Nanobuds were first reported in 2007 and are hybrid buckytube/buckyball materials (buckyballs are covalently bonded to the outer wall of a nanotube) that combine the properties of both in a single structure.
Of the other discovered allotropes, carbon nanofoam is a ferromagnetic allotrope discovered in 1997. It consists of a low-density cluster-assembly of carbon atoms strung together in a loose three-dimensional web, in which the atoms are bonded trigonally in six- and seven-membered rings. It is among the lightest known solids, with a density of about 2 kg/m. Similarly, glassy carbon contains a high proportion of closed porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
, but contrary to normal graphite, the graphitic layers are not stacked like pages in a book, but have a more random arrangement. Linear acetylenic carbon
Linear acetylenic carbon (LAC), also known as carbyne or a Linear Carbon Chain (LCC), is an Allotropes of carbon, allotrope of carbon that has the chemical structure as a repeat unit, with alternating Single bond, single and triple bonds. It w ...
has the chemical structure −(C≡C)− . Carbon in this modification is linear with ''sp'' orbital hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
, and is a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
with alternating single and triple bonds. This carbyne is of considerable interest to nanotechnology
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing propertie ...
as its Young's modulus
Young's modulus (or the Young modulus) is a mechanical property of solid materials that measures the tensile or compressive stiffness when the force is applied lengthwise. It is the modulus of elasticity for tension or axial compression. Youn ...
is 40 times that of the hardest known material – diamond.
In 2015, a team at the North Carolina State University
North Carolina State University (NC State, North Carolina State, NC State University, or NCSU) is a public university, public Land-grant university, land-grant research university in Raleigh, North Carolina, United States. Founded in 1887 and p ...
announced the development of another allotrope they have dubbed Q-carbon, created by a high-energy low-duration laser pulse on amorphous carbon dust. Q-carbon is reported to exhibit ferromagnetism, fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colore ...
, and a hardness superior to diamonds.
Isotopes
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s of carbon are atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. Aft ...
that contain six proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s plus a number of neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
s (varying from 2 to 16). Carbon has two stable, naturally occurring isotopes. The isotope carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-1 ...
(C) forms 98.93% of the carbon on Earth, while carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth.
Detection by mass spectrometry
A m ...
(C) forms the remaining 1.07%. The concentration of C is further increased in biological materials because biochemical reactions discriminate against C. In 1961, the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) adopted the isotope carbon-12 as the basis for atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
s. Identification of carbon in nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR) experiments is done with the isotope C.
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and coll ...
(C) is a naturally occurring radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ...
, created in the upper atmosphere
Upper atmosphere is a collective term that refers to various layers of the atmosphere of the Earth above the troposphere and corresponding regions of the atmospheres of other planets, and includes:
* The mesosphere, which on Earth lies between th ...
(lower stratosphere
The stratosphere () is the second-lowest layer of the atmosphere of Earth, located above the troposphere and below the mesosphere. The stratosphere is composed of stratified temperature zones, with the warmer layers of air located higher ...
and upper troposphere
The troposphere is the lowest layer of the atmosphere of Earth. It contains 80% of the total mass of the Atmosphere, planetary atmosphere and 99% of the total mass of water vapor and aerosols, and is where most weather phenomena occur. From the ...
) by interaction of nitrogen with cosmic rays. It is found in trace amounts on Earth of 1 part per trillion
''Trillion'' is a number with two distinct definitions:
*1,000,000,000,000, i.e. one million 1,000,000, million, or (ten to the twelfth Exponentiation, power), as defined on the long and short scales, short scale. This is now the meaning in bot ...
(0.0000000001%) or more, mostly confined to the atmosphere and superficial deposits, particularly of peat and other organic materials. This isotope decays by 0.158 MeV β emission. Because of its relatively short half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of years, C is virtually absent in ancient rocks. The amount of C in the atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
and in living organisms is almost constant, but decreases predictably in their bodies after death. This principle is used in radiocarbon dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for Chronological dating, determining the age of an object containing organic material by using the properties of carbon-14, radiocarbon, a radioactive Isotop ...
, invented in 1949, which has been used extensively to determine the age of carbonaceous materials with ages up to about 40,000 years.
There are 15 known isotopes of carbon and the shortest-lived of these is C which decays through proton emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a atomic nucleus, nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay ...
and has a half-life of 3.5 s. The exotic C exhibits a nuclear halo, which means its radius is appreciably larger than would be expected if the nucleus were a sphere of constant density.
Occurrence

 Carbon is the fourth most abundant chemical element in the observable universe by mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets. Some
Carbon is the fourth most abundant chemical element in the observable universe by mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets. Some meteorite
A meteorite is a rock (geology), rock that originated in outer space and has fallen to the surface of a planet or Natural satellite, moon. When the original object enters the atmosphere, various factors such as friction, pressure, and chemical ...
s contain microscopic diamonds that were formed when the Solar System was still a protoplanetary disk
A protoplanetary disk is a rotating circumstellar disc of dense gas and dust surrounding a young newly formed star, a T Tauri star, or Herbig Ae/Be star. The protoplanetary disk may not be considered an accretion disk; while the two are sim ...
. Microscopic diamonds may also be formed by the intense pressure and high temperature at the sites of meteorite impacts.
In 2014 NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the federal government of the United States, US federal government responsible for the United States ...
announced greatly upgraded database
for tracking
polycyclic aromatic hydrocarbons
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incin ...
(PAHs) in the universe. More than 20% of the carbon in the universe may be associated with PAHs, complex compounds of carbon and hydrogen without oxygen. These compounds figure in the PAH world hypothesis where they are hypothesized to have a role in abiogenesis
Abiogenesis is the natural process by which life arises from non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to living entities on Earth was not a single even ...
and formation of life. PAHs seem to have been formed "a couple of billion years" after the Big Bang
The Big Bang is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models based on the Big Bang concept explain a broad range of phenomena, including th ...
, are widespread throughout the universe, and are associated with new stars and exoplanet
An exoplanet or extrasolar planet is a planet outside the Solar System. The first confirmed detection of an exoplanet was in 1992 around a pulsar, and the first detection around a main-sequence star was in 1995. A different planet, first det ...
s.
It has been estimated that the solid earth as a whole contains 730 ppm of carbon, with 2000 ppm in the core and 120 ppm in the combined mantle and crust. Since the mass of the earth is , this would imply 4360 million gigatonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the sh ...
s of carbon. This is much more than the amount of carbon in the oceans or atmosphere (below).
In combination with oxygen in carbon dioxide, carbon is found in the Earth's atmosphere (approximately 900 gigatonnes of carbon — each ppm corresponds to 2.13 Gt) and dissolved in all water bodies (approximately 36,000 gigatonnes of carbon). Carbon in the biosphere
The biosphere (), also called the ecosphere (), is the worldwide sum of all ecosystems. It can also be termed the zone of life on the Earth. The biosphere (which is technically a spherical shell) is virtually a closed system with regard to mat ...
has been estimated at 550 gigatonnes but with a large uncertainty, due mostly to a huge uncertainty in the amount of terrestrial deep subsurface bacteria. Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may b ...
(such as coal, petroleum, and natural gas) contain carbon as well. Coal "reserves" (not "resources") amount to around 900 gigatonnes with perhaps 18,000 Gt of resources. Oil reserves
An oil is any chemical polarity, nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobe, hydrophobic (does not mix with water) and lipophilicity, lipophilic (mixes with other oils). Oils are usually flammable ...
are around 150 gigatonnes. Proven sources of natural gas are about (containing about 105 gigatonnes of carbon), but studies estimate another of "unconventional" deposits such as shale gas
Shale gas is an unconventional natural gas that is found trapped within shale formations. Since the 1990s, a combination of horizontal drilling and hydraulic fracturing has made large volumes of shale gas more economical to produce, and ...
, representing about 540 gigatonnes of carbon.
Carbon is also found in methane hydrates
Methane clathrate (CH4·5.75H2O) or (4CH4·23H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large am ...
in polar regions and under the seas. Various estimates put this carbon between 500, 2500, or 3,000 Gt.
According to one source, in the period from 1751 to 2008 about 347 gigatonnes of carbon were released as carbon dioxide to the atmosphere from burning of fossil fuels. Another source puts the amount added to the atmosphere for the period since 1750 at 879 Gt, and the total going to the atmosphere, sea, and land (such as peat bog
A bog or bogland is a wetland that accumulates peat as a deposit of dead plant materials often mosses, typically sphagnum moss. It is one of the four main types of wetlands. Other names for bogs include mire, mosses, quagmire, and muske ...
s) at almost 2,000 Gt.
Carbon is a constituent (about 12% by mass) of the very large masses of carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
rock (limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
, dolomite, marble
Marble is a metamorphic rock consisting of carbonate minerals (most commonly calcite (CaCO3) or Dolomite (mineral), dolomite (CaMg(CO3)2) that have recrystallized under the influence of heat and pressure. It has a crystalline texture, and is ty ...
, and others). Coal is very rich in carbon (anthracite
Anthracite, also known as hard coal and black coal, is a hard, compact variety of coal that has a lustre (mineralogy)#Submetallic lustre, submetallic lustre. It has the highest carbon content, the fewest impurities, and the highest energy densit ...
contains 92–98%) and is the largest commercial source of mineral carbon, accounting for 4,000 gigatonnes or 80% of fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
.
As for individual carbon allotropes, graphite is found in large quantities in China, Russia, Mexico, Canada, and India. Natural diamonds occur in the rock kimberlite
Kimberlite is an igneous rock and a rare variant of peridotite. It is most commonly known as the main host matrix for diamonds. It is named after the town of Kimberley, Northern Cape, Kimberley in South Africa, where the discovery of an 83.5-Car ...
, found in ancient volcanic "necks", or "pipes". Most diamond deposits are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the Congo, and Angola. Diamond deposits have also been found in Arkansas
Arkansas ( ) is a landlocked state in the West South Central region of the Southern United States. It borders Missouri to the north, Tennessee and Mississippi to the east, Louisiana to the south, Texas to the southwest, and Oklahoma ...
, Canada, the Russian Arctic, Brazil, and in Northern and Western Australia. Diamonds are found naturally, but about 90% of all industrial diamonds used in the U.S. are now manufactured.
Carbon-14 is formed in upper layers of the troposphere and the stratosphere at altitudes of 9–15 km by a reaction that is precipitated by cosmic ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the ...
s. Thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium wit ...
s are produced that collide with the nuclei of nitrogen-14, forming carbon-14 and a proton. As such, of atmospheric carbon dioxide contains carbon-14.
Carbon-rich asteroids are relatively preponderant in the outer parts of the asteroid belt
The asteroid belt is a torus-shaped region in the Solar System, centered on the Sun and roughly spanning the space between the orbits of the planets Jupiter and Mars. It contains a great many solid, irregularly shaped bodies called asteroids ...
in the Solar System. These asteroids have not yet been directly sampled by scientists. The asteroids can be used in hypothetical space-based carbon mining, which may be possible in the future, but is currently technologically impossible.
Formation in stars
Formation of the carbon atomic nucleus occurs within agiant
In folklore, giants (from Ancient Greek: ''wiktionary:gigas, gigas'', cognate wiktionary:giga-, giga-) are beings of humanoid appearance, but are at times prodigious in size and strength or bear an otherwise notable appearance. The word ''gia ...
or supergiant
Supergiants are among the most massive and most luminous stars. Supergiant stars occupy the top region of the Hertzsprung–Russell diagram, with absolute visual magnitudes between about −3 and −8. The temperatures of supergiant stars range ...
star through the triple-alpha process
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.
In stars
Helium accumulates in the cores of stars as a result of the proton–proton chain reaction a ...
. This requires a nearly simultaneous collision of three alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s (helium nuclei), as the products of further nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
reactions of helium with hydrogen or another helium nucleus produce lithium-5
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 (6Li) and lithium-7 (7Li), with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucle ...
and beryllium-8
Beryllium-8 (8Be, Be-8) is a radionuclide with 4 neutrons and 4 protons. It is an unbound resonance and nominally an isotope of beryllium. It has a half-life on the order of 8.19 seconds, decaying into two alpha particles. This has importa ...
respectively, both of which are highly unstable and decay almost instantly back into smaller nuclei. The triple-alpha process happens in conditions of temperatures over 100 megakelvins and helium concentration that the rapid expansion and cooling of the early universe prohibited, and therefore no significant carbon was created during the Big Bang.
According to current physical cosmology theory, carbon is formed in the interiors of stars on the horizontal branch
The horizontal branch (HB) is a stage of stellar evolution that immediately follows the red-giant branch in stars whose masses are similar to the Sun's. Horizontal-branch stars are powered by helium fusion in the core (via the triple-alpha proc ...
. When massive stars die as supernova, the carbon is scattered into space as dust. This dust becomes component material for the formation of the next-generation star systems with accreted planets. The Solar System is one such star system with an abundance of carbon, enabling the existence of life as we know it. It is the opinion of most scholars that all the carbon in the Solar System and the Milky Way
The Milky Way or Milky Way Galaxy is the galaxy that includes the Solar System, with the name describing the #Appearance, galaxy's appearance from Earth: a hazy band of light seen in the night sky formed from stars in other arms of the galax ...
comes from dying stars.
The CNO cycle
In astrophysics, the carbon–nitrogen–oxygen (CNO) cycle, sometimes called Bethe–Weizsäcker cycle, after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker, is one of the two known sets of fusion reactions by which stars convert h ...
is an additional hydrogen fusion mechanism that powers stars, wherein carbon operates as a catalyst.
Rotational transitions of various isotopic forms of carbon monoxide (for example, CO, CO, and CO) are detectable in the submillimeter
Submillimetre astronomy or submillimeter astronomy (see spelling differences) is the branch of observational astronomy that is conducted at submillimetre wavelengths (i.e., terahertz radiation) of the electromagnetic spectrum. Astronomers plac ...
wavelength range, and are used in the study of newly forming stars in molecular cloud
A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...
s.
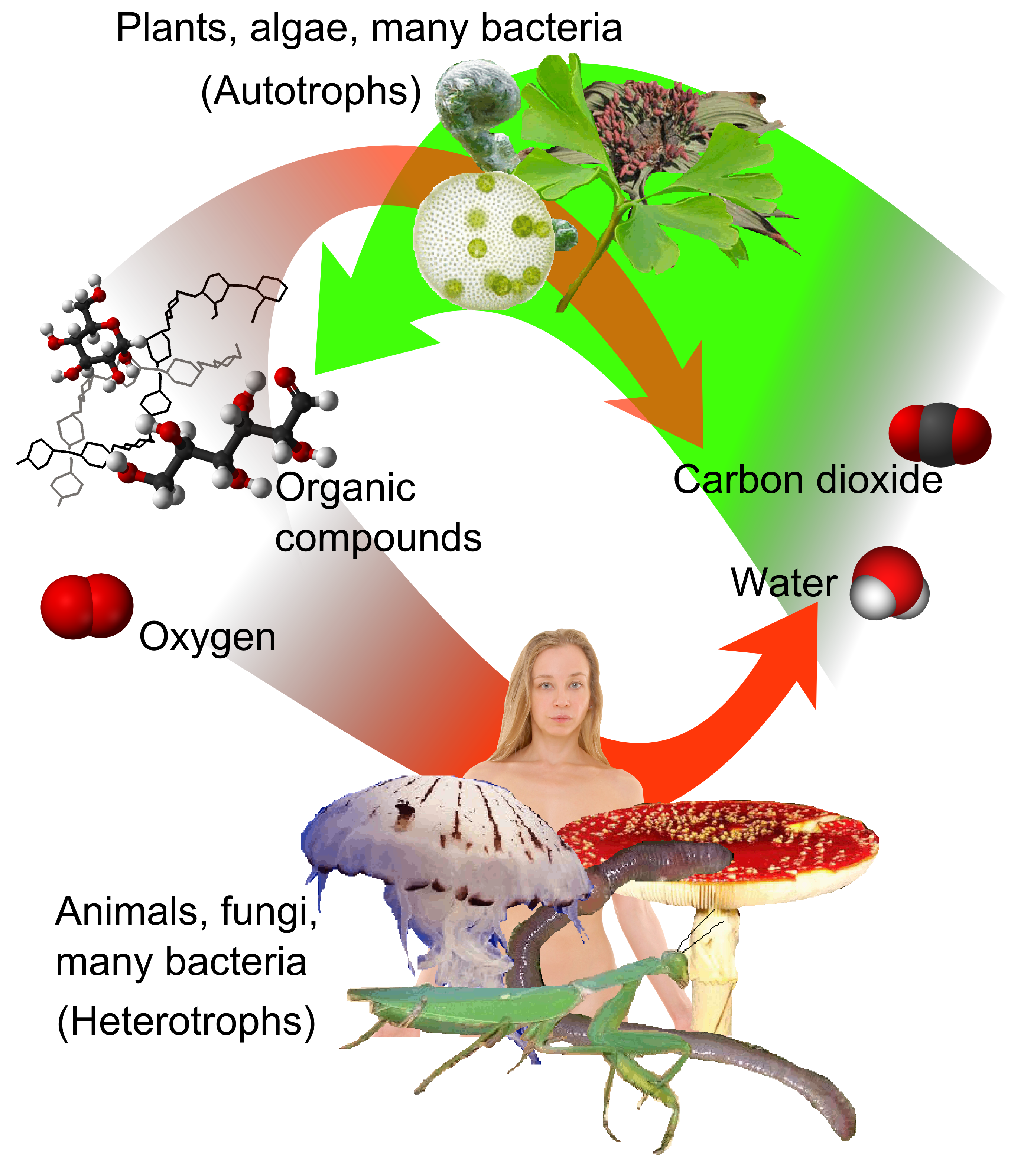
Carbon cycle
carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
. For example, photosynthetic
Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ...
plants draw carbon dioxide from the atmosphere (or seawater) and build it into biomass, as in the Calvin cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into ...
, a process of carbon fixation
Biological carbon fixation, or сarbon assimilation, is the Biological process, process by which living organisms convert Total inorganic carbon, inorganic carbon (particularly carbon dioxide, ) to Organic compound, organic compounds. These o ...
. Some of this biomass is eaten by animals, while some carbon is exhaled by animals as carbon dioxide. The carbon cycle is considerably more complicated than this short loop; for example, some carbon dioxide is dissolved in the oceans; if bacteria do not consume it, dead plant or animal matter may become petroleum or coal, which releases carbon when burned.
Compounds
Organic compounds
 Carbon can form very long chains of interconnecting
Carbon can form very long chains of interconnecting carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
s, a property that is called catenation
In chemistry, catenation is the chemical bond, bonding of atoms of the same Chemical element, element into a series, called a ''chain''. A chain or a Ring (chemistry), ring may be ''open'' if its ends are not bonded to each other (an open-chain c ...
. Carbon-carbon bonds are strong and stable. Through [p[catenation, carbon forms a countless number of compounds. A tally of unique compounds shows that more contain carbon than do not.
The simplest form of an organic molecule is the hydrocarbon—a large family of organic molecules that are composed of hydrogen atoms bonded to a chain of carbon atoms. A hydrocarbon backbone can be substituted by other atoms, known as heteroatoms. Common heteroatoms that appear in organic compounds include oxygen, nitrogen, sulfur, phosphorus, and the nonradioactive halogens, as well as the metals lithium and magnesium. Organic compounds containing bonds to metal are known as organometallic compounds (''see below''). Certain groupings of atoms, often including heteroatoms, recur in large numbers of organic compounds. These collections, known as ''functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s'', confer common reactivity patterns and allow for the systematic study and categorization of organic compounds. Chain length, shape and functional groups all affect the properties of organic molecules.
In most stable compounds of carbon (and nearly all stable ''organic'' compounds), carbon obeys the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
and is ''tetravalent'', meaning that a carbon atom forms a total of four covalent bonds (which may include double and triple bonds). Exceptions include a small number of stabilized ''carbocations'' (three bonds, positive charge), ''radicals'' (three bonds, neutral), ''carbanions'' (three bonds, negative charge) and ''carbenes'' (two bonds, neutral), although these species are much more likely to be encountered as unstable, reactive intermediates.
Carbon occurs in all known organic life and is the basis of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. When united with hydrogen, it forms various hydrocarbons that are important to industry as refrigerants, lubricants, solvents, as chemical feedstock for the manufacture of plastics and petrochemicals, and as fossil fuels.
When combined with oxygen and hydrogen, carbon can form many groups of important biological compounds including sugars, lignan
The lignans are a large group of low molecular weight polyphenols found in plants, particularly seeds, whole grains, and vegetables. The name derives from the Latin word for "wood". Lignans are precursors to phytoestrogens. They may play a rol ...
s, chitin
Chitin (carbon, C8hydrogen, H13oxygen, O5nitrogen, N)n ( ) is a long-chain polymer of N-Acetylglucosamine, ''N''-acetylglucosamine, an amide derivative of glucose. Chitin is the second most abundant polysaccharide in nature (behind only cell ...
s, alcohols, fats, aromatic ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, carotenoid
Carotenoids () are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, cana ...
s and terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
s. With nitrogen, it forms alkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s, and with the addition of sulfur also it forms antibiotics, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s, and rubber products. With the addition of phosphorus to these other elements, it forms DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
and RNA
Ribonucleic acid (RNA) is a polymeric molecule that is essential for most biological functions, either by performing the function itself (non-coding RNA) or by forming a template for the production of proteins (messenger RNA). RNA and deoxyrib ...
, the chemical-code carriers of life, and adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP), the most important energy-transfer molecule in all living cells. Norman Horowitz
Norman Harold Horowitz (March 19, 1915 – June 1, 2005) was an American geneticist at Caltech who achieved national fame as the scientist who devised experiments to determine whether life might exist on Mars. His experiments were carried out by ...
, head of the Mariner and Viking missions to Mars (1965–1976), considered that the unique characteristics of carbon made it unlikely that any other element could replace carbon, even on another planet, to generate the biochemistry necessary for life.
Inorganic compounds
Commonly carbon-containing compounds which are associated with minerals or which do not contain bonds to the other carbon atoms, halogens, or hydrogen, are treated separately from classical organic compounds; the definition is not rigid, and the classification of some compounds can vary from author to author (see reference articles above). Among these are the simple oxides of carbon. The most prominent oxide is carbon dioxide (). This was once the principal constituent of thepaleoatmosphere
A paleoatmosphere (or ''palaeoatmosphere'') is an atmosphere, particularly that of Earth, at some unspecified time in the geological past.
When regarding geological history of Earth, the paleoatmosphere can be chronologically divided into the H ...
, but is a minor component of the Earth's atmosphere
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weathe ...
today. Dissolved in water, it forms carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
(), but as most compounds with multiple single-bonded oxygens on a single carbon it is unstable. Through this intermediate, though, resonance-stabilized carbonate ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s are produced. Some important minerals are carbonates, notably calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
. Carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
() is similar.
The other common oxide is carbon monoxide (CO). It is formed by incomplete combustion, and is a colorless, odorless gas. The molecules each contain a triple bond and are fairly polar, resulting in a tendency to bind permanently to hemoglobin molecules, displacing oxygen, which has a lower binding affinity. Cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
(CN), has a similar structure, but behaves much like a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
ion (pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
). For example, it can form the nitride cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a ...
molecule ((CN)), similar to diatomic halides. Likewise, the heavier analog of cyanide, cyaphide (CP), is also considered inorganic, though most simple derivatives are highly unstable. Other uncommon oxides are carbon suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , wh ...
(), the unstable dicarbon monoxide (CO), carbon trioxide (CO), cyclopentanepentone (CO), cyclohexanehexone (CO), and mellitic anhydride
Mellitic anhydride, the anhydride of mellitic acid, is an organic compound with the formula C12O9.
Containing no other elements (e.g., hydrogen) besides carbon and oxygen, mellitic anhydride is an oxide of carbon (oxocarbon), and, along with CO ...
(CO). However, mellitic anhydride is the triple acyl anhydride of mellitic acid; moreover, it contains a benzene ring. Thus, many chemists consider it to be organic.
With reactive metals, such as tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, carbon forms either carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s (C) or acetylide
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) by metallic or other cations. Calcium carbide is an important industrial compound, which has long been used ...
s () to form alloys with high melting points. These anions are also associated with methane and acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
, both very weak acids. With an electronegativity of 2.5, carbon prefers to form covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s. A few carbides are covalent lattices, like carborundum
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder ...
(SiC), which resembles diamond. Nevertheless, even the most polar and salt-like of carbides are not completely ionic compounds.
Organometallic compounds
Organometallic compounds by definition contain at least one carbon-metal covalent bond. A wide range of such compounds exist; major classes include simple alkyl-metal compounds (for example,tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
), η-alkene compounds (for example, Zeise's salt
Zeise's salt, potassium trichloro(ethylene)platinate(II) hydrate, is the chemical compound with the formula K platinum.html" ;"title="/nowiki>platinum">PtCl3(C2H4)�H2O. The anion of this air-stable, yellow, coordination complex contains an hapt ...
), and η-allyl compounds (for example, allylpalladium chloride dimer); metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
s containing cyclopentadienyl ligands (for example, ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
); and transition metal carbene complex
A transition metal carbene complex is an organometallic compound featuring a divalent carbon ligand, itself also called a carbene. Carbene complexes have been synthesized from most transition metals and f-block metals, using many different synt ...
es. Many metal carbonyl
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against n ...
s and metal cyanides exist (for example, tetracarbonylnickel
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a Organonickel chemistry, nickel(0) organometallic compound with the chemical formula, formula Ni(CO)4. This colorless liquid is the principal metal carbonyl, carbonyl of nickel. It is an React ...
and potassium ferricyanide
Potassium ferricyanide is the chemical compound with the formula K3 e(CN)6 This bright red salt contains the octahedral molecular geometry, octahedrally coordination compound, coordinated ferricyanide, e(CN)6− ion. It is soluble in wat ...
); some workers consider metal carbonyl and cyanide complexes without other carbon ligands to be purely inorganic, and not organometallic. However, most organometallic chemists consider metal complexes with any carbon ligand, even 'inorganic carbon' (e.g., carbonyls, cyanides, and certain types of carbides and acetylides) to be organometallic in nature. Metal complexes containing organic ligands without a carbon-metal covalent bond (e.g., metal carboxylates) are termed ''metalorganic'' compounds.
While carbon is understood to strongly prefer formation of four covalent bonds, other exotic bonding schemes are also known. Carborane
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron ...
s are highly stable dodecahedral derivatives of the 12H12sup>2- unit, with one BH replaced with a CH+. Thus, the carbon is bonded to five boron atoms and one hydrogen atom. The cation PhPAu)Ccontains an octahedral carbon bound to six phosphine-gold fragments. This phenomenon has been attributed to the aurophilicity
In chemistry, aurophilicity refers to the tendency of gold complexes to aggregate via formation of weak metallophilic interactions.
The main evidence for aurophilicity is from the crystallographic analysis of Au(I) complexes. The aurophilic bo ...
of the gold ligands, which provide additional stabilization of an otherwise labile species. In nature, the iron-molybdenum cofactor (FeMoco
FeMoco ( cofactor) or M-cluster is the primary cofactor of nitrogenase. Nitrogenase is the enzyme that catalyzes the conversion of atmospheric nitrogen molecules N2 into ammonia (NH3) through the process known as nitrogen fixation. Because it con ...
) responsible for microbial nitrogen fixation
Nitrogen fixation is a chemical process by which molecular dinitrogen () is converted into ammonia (). It occurs both biologically and abiological nitrogen fixation, abiologically in chemical industry, chemical industries. Biological nitrogen ...
likewise has an octahedral carbon center (formally a carbide, C(-IV)) bonded to six iron atoms. In 2016, it was confirmed that, in line with earlier theoretical predictions, the hexamethylbenzene dication contains a carbon atom with six bonds. More specifically, the dication could be described structurally by the formulation eC(η5-C5Me5)sup>2+, making it an "organic metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
" in which a MeC3+ fragment is bonded to a η5-C5Me5− fragment through all five of the carbons of the ring.
It is important to note that in the cases above, each of the bonds to carbon contain less than two formal electron pairs. Thus, the formal electron count of these species does not exceed an octet. This makes them hypercoordinate but not hypervalent. Even in cases of alleged 10-C-5 species (that is, a carbon with five ligands and a formal electron count of ten), as reported by Akiba and co-workers, electronic structure calculations conclude that the electron population around carbon is still less than eight, as is true for other compounds featuring four-electron three-center bonding.
History and etymology
 The English name ''carbon'' comes from the Latin ''carbo'' for coal and charcoal, whence also comes the French ''charbon'', meaning charcoal. In German, Dutch and Danish, the names for carbon are ''Kohlenstoff'', ''koolstof'', and ''kulstof'' respectively, all literally meaning coal-substance.
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.
The English name ''carbon'' comes from the Latin ''carbo'' for coal and charcoal, whence also comes the French ''charbon'', meaning charcoal. In German, Dutch and Danish, the names for carbon are ''Kohlenstoff'', ''koolstof'', and ''kulstof'' respectively, all literally meaning coal-substance.
Carbon was discovered in prehistory and was known in the forms of soot and charcoal to the earliest human civilizations. Diamonds were known probably as early as 2500 BCE in China, while carbon in the form of charcoal was made by the same chemistry as it is today, by heating wood in a pyramid covered with clay to exclude air.
 In 1722, René Antoine Ferchault de Réaumur demonstrated that iron was transformed into steel through the absorption of some substance, now known to be carbon. In 1772,
In 1722, René Antoine Ferchault de Réaumur demonstrated that iron was transformed into steel through the absorption of some substance, now known to be carbon. In 1772, Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
showed that diamonds are a form of carbon; when he burned samples of charcoal and diamond and found that neither produced any water and that both released the same amount of carbon dioxide per gram. In 1779, Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish Pomerania, German-Swedish pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified the elements molybd ...
showed that graphite, which had been thought of as a form of lead, was instead identical with charcoal but with a small admixture of iron, and that it gave "aerial acid" (his name for carbon dioxide) when oxidized with nitric acid. In 1786, the French scientists Claude Louis Berthollet
Claude Louis Berthollet (, 9 December 1748 – 6 November 1822) was a Savoyard-French chemist who became vice president of the French Senate in 1804. He is known for his scientific contributions to the theory of chemical equilibria via the ...
, Gaspard Monge
Gaspard Monge, Comte de Péluse (; 9 May 1746 – 28 July 1818) was a French mathematician, commonly presented as the inventor of descriptive geometry, (the mathematical basis of) technical drawing, and the father of differential geometry. Dur ...
and C. A. Vandermonde confirmed that graphite was mostly carbon by oxidizing it in oxygen in much the same way Lavoisier had done with diamond. Some iron again was left, which the French scientists thought was necessary to the graphite structure. In their publication they proposed the name ''carbone'' (Latin ''carbonum'') for the element in graphite which was given off as a gas upon burning graphite. Antoine Lavoisier then listed carbon as an element in his 1789 textbook.
A new allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
of carbon, fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
, that was discovered in 1985 includes nanostructure
A nanostructure is a structure of intermediate size between microscopic and molecular structures. Nanostructural detail is microstructure at nanoscale.
In describing nanostructures, it is necessary to differentiate between the number of dimen ...
d forms such as buckyball
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to i ...
s and nanotubes. Their discoverers – Robert Curl
Robert Floyd Curl Jr. (August 23, 1933 – July 3, 2022) was an American chemist who was Pitzer–Schlumberger Professor of Natural Sciences and professor of chemistry at Rice University. He was awarded the Nobel Prize in Chemistry in 1996 for ...
, Harold Kroto
Sir Harold Walter Kroto (born Harold Walter Krotoschiner; 7 October 1939 – 30 April 2016) was an English chemist. He shared the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley for their discovery of fullerenes. He was th ...
, and Richard Smalley
Richard Errett Smalley (June 6, 1943 – October 28, 2005) was an American chemist who was the Gene and Norman Hackerman Professor of Chemistry, Physics, and Astronomy at Rice University. In 1996, along with Robert Curl, also a professor of ...
– received the Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
in Chemistry in 1996. The resulting renewed interest in new forms led to the discovery of further exotic allotropes, including glassy carbon, and the realization that "amorphous carbon
Amorphous carbon is free, reactive carbon that has no crystalline structure. Amorphous carbon materials may be stabilized by terminating dangling-π bonds with hydrogen. As with other amorphous solids, some short-range order can be observed. Amo ...
" is not strictly amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
.
Production
Graphite
Commercially viable natural deposits of graphite occur in many parts of the world, but the most important sources economically are in China, India, Brazil, and North Korea. Graphite deposits are ofmetamorphic
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock (protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, causi ...
origin, found in association with quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
, mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect basal cleavage''. Mica is co ...
, and feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagiocl ...
s in schists, gneiss
Gneiss (pronounced ) is a common and widely distributed type of metamorphic rock. It is formed by high-temperature and high-pressure metamorphic processes acting on formations composed of igneous or sedimentary rocks. This rock is formed under p ...
es, and metamorphosed sandstone
Sandstone is a Clastic rock#Sedimentary clastic rocks, clastic sedimentary rock composed mainly of grain size, sand-sized (0.0625 to 2 mm) silicate mineral, silicate grains, Cementation (geology), cemented together by another mineral. Sand ...
s and limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science) ...
as lenses
A lens is a transmissive optical device that focuses or disperses a light beam by means of refraction. A simple lens consists of a single piece of transparent material, while a compound lens consists of several simple lenses (''elements''), ...
or veins
Veins () are blood vessels in the circulatory system of humans and most other animals that carry blood towards the heart. Most veins carry deoxygenated blood from the tissues back to the heart; exceptions are those of the pulmonary and fetal c ...
, sometimes of a metre or more in thickness. Deposits of graphite in Borrowdale
Borrowdale is a valley and civil parish in the English Lake District in Cumberland (unitary authority), Cumberland, England. It is in the ceremonial county of Cumbria, and is sometimes referred to as ''Cumberland Borrowdale'' to distinguis ...
, Cumberland
Cumberland ( ) is an area of North West England which was historically a county. The county was bordered by Northumberland to the north-east, County Durham to the east, Westmorland to the south-east, Lancashire to the south, and the Scottish ...
, England were at first of sufficient size and purity that, until the 19th century, pencils were made by sawing blocks of natural graphite into strips before encasing the strips in wood. Today, smaller deposits of graphite are obtained by crushing the parent rock and floating the lighter graphite out on water.USGS Minerals Yearbook: Graphite, 2009and Graphite: Mineral Commodity Summaries 2011 There are three types of natural graphite—amorphous, flake or crystalline flake, and vein or lump. Amorphous graphite is the lowest quality and most abundant. Contrary to science, in industry "amorphous" refers to very small crystal size rather than complete lack of crystal structure. Amorphous is used for lower value graphite products and is the lowest priced graphite. Large amorphous graphite deposits are found in China, Europe, Mexico and the United States. Flake graphite is less common and of higher quality than amorphous; it occurs as separate plates that crystallized in metamorphic rock. Flake graphite can be four times the price of amorphous. Good quality flakes can be processed into expandable graphite for many uses, such as
flame retardant
Flame retardants are a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an combustion, ignition source and pr ...
s. The foremost deposits are found in Austria, Brazil, Canada, China, Germany and Madagascar. Vein or lump graphite is the rarest, most valuable, and highest quality type of natural graphite. It occurs in veins along intrusive contacts in solid lumps, and it is only commercially mined in Sri Lanka.
According to the USGS
The United States Geological Survey (USGS), founded as the Geological Survey, is an government agency, agency of the United States Department of the Interior, U.S. Department of the Interior whose work spans the disciplines of biology, geograp ...
, world production of natural graphite was 1.1 million tonnes in 2010, to which China contributed 800,000 t, India 130,000 t, Brazil 76,000 t, North Korea 30,000 t and Canada 25,000 t. No natural graphite was reported mined in the United States, but 118,000 t of synthetic graphite with an estimated value of $998 million was produced in 2009.
Diamond
 The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of
The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care has to be taken in order to prevent larger diamonds from being destroyed in this process and subsequently the particles are sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence
X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis ...
, after which the final sorting steps are done by hand. Before the use of X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
s became commonplace, the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.
Historically diamonds were known to be found only in alluvial deposits in southern India. discussion on alluvial diamonds in India and elsewhere as well as earliest finds India led the world in diamond production from the time of their discovery in approximately the 9th century BC Ball was a Geologist in British service. Chapter I, Page 1 to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.
Diamond production of primary deposits (kimberlites and lamproites) only started in the 1870s after the discovery of the diamond fields in South Africa. Production has increased over time and an accumulated total of over 4.5 billion carats have been mined since that date. Most commercially viable diamond deposits were in Russia, Botswana, Australia and the Democratic Republic of Congo. By 2005, Russia produced almost one-fifth of the global diamond output (mostly in Yakutia territory; for example, Mir pipe and Udachnaya pipe
The Udachnaya pipe (, ; ) is a diamond deposit in the Daldyn- Alakit kimberlite field in Sakha Republic, Russia. It is an open-pit mine, and is located just outside the Arctic Circle at .
History
Udachnaya was discovered on 15 June 1955, just two ...
) but the Argyle mine in Australia became the single largest source, producing 14 million carats in 2018. New finds, the Canadian mines at Diavik and Ekati
The Ekati Diamond Mine, often simply called Ekati, is Canada's first surface and underground diamond mine and is owned by Burgundy Diamond Mines. It is located north-east of Yellowknife, Northwest Territories, and about south of the Arctic C ...
, are expected to become even more valuable owing to their production of gem quality stones.
In the United States, diamonds have been found in Arkansas, Colorado, and Montana. In 2004, a startling discovery of a microscopic diamond in the United States led to the January 2008 bulk-sampling of kimberlite pipes in a remote part of Montana.
While natural diamonds form over time deep within the Earth, synthetic diamonds are created in laboratories through a variety of methods. The original method uses high pressure and high temperature (HPHT) and is still widely used because of its relatively low cost. The process involves large presses that can weigh hundreds of tons to produce a pressure of at . The second method, using chemical vapor deposition (CVD), creates a carbon plasma over a substrate onto which the carbon atoms deposit to form diamond. Other methods include explosive formation (forming detonation nanodiamonds) and sonication
image:Sonicator.jpg, A sonicator at the Weizmann Institute of Science during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, ...
of graphite solutions.
Applications
Carbon is essential to all known living systems, and without it life as we know it could not exist (seealternative biochemistry
Several forms of biochemistry are agreed to be scientifically viable but are not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as ...
). The major economic use of carbon other than food and wood is in the form of hydrocarbons, most notably the fossil fuel methane gas and crude oil (petroleum). Crude oil is distilled in refineries by the petrochemical industry
file:Jampilen Petrochemical Co. 02.jpg, 300px, Jampilen Petrochemical co., Asaluyeh, Iran
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics industry, plastics (poly ...
to produce gasoline, kerosene, and other products. Cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
is a natural, carbon-containing polymer produced by plants in the form of wood, cotton, linen, and hemp
Hemp, or industrial hemp, is a plant in the botanical class of ''Cannabis sativa'' cultivars grown specifically for industrial and consumable use. It can be used to make a wide range of products. Along with bamboo, hemp is among the fastest ...
. Cellulose is used primarily for maintaining structure in plants. Commercially valuable carbon polymers of animal origin include wool, cashmere, and silk. Plastics are made from synthetic carbon polymers, often with oxygen and nitrogen atoms included at regular intervals in the main polymer chain. The raw materials for many of these synthetic substances come from crude oil and coal.
The uses of carbon and its compounds are extremely varied. It can form alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
s with iron, of which the most common is carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
. Graphite is combined with clays to form the 'lead' used in pencils used for writing and drawing. It is also used as a lubricant and a pigment, as a moulding material in glass manufacture, in electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s for dry batteries and in electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
and electroforming
Electroforming is a metal forming process in which parts are fabricated through electrodeposition on a model, known in the industry as a mandrel. Conductive (metallic) mandrels are treated to create a mechanical parting layer, or are chemicall ...
, in brushes
A brush is a common tool with bristles, wire or other filaments. It generally consists of a handle or block to which filaments are affixed in either a parallel or perpendicular orientation, depending on the way the brush is to be gripped during u ...
for electric motors
An electric motor is a machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a wire winding to generate Laplace force i ...
, and as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons ...
s.
Charcoal is used as a drawing material in artwork
A work of art, artwork, art piece, piece of art or art object is an artistic creation of aesthetic value. Except for "work of art", which may be used of any work regarded as art in its widest sense, including works from literature ...
, barbecue grilling, iron smelting
Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron, copper, silver, tin, lead and zinc ...
, and in many other applications. Wood, coal and oil are used as fuel for production of energy and heating. Gem quality diamond is used in jewelry, and industrial diamonds are used in drilling, cutting and polishing tools for machining metals and stone. Carbon fiber
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers ( Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon comp ...
, which is produced by pyrolyzing synthetic polyester fibers, is used to reinforce plastics, creating advanced, lightweight composite materials.
Carbon fiber is made by pyrolysis of extruded and stretched filaments of polyacrylonitrile
Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (CH2CHCN)n. Almost all PAN resins are copolymers with acrylonitrile as the main monomer. PAN is used to produce large variety of products in ...
(PAN) and other organic substances. The crystallographic structure and mechanical properties of the fiber depend on the type of starting material, and on the subsequent processing. Carbon fibers made from PAN have structure resembling narrow filaments of graphite, but thermal processing may re-order the structure into a continuous rolled sheet. The result is fibers with higher specific tensile strength than steel.
Carbon black
Carbon black (with subtypes acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal tar, vegetable matter, or petroleum products, including fuel oil, fluid cataly ...
is used as the black pigment in printing ink, artist's oil paint, and water colours, carbon paper
Carbon paper (originally carbonic paper) consists of sheets of paper that create one or more copies simultaneously with the creation of an original document when inscribed by a typewriter or ballpoint pen. The email term cc which means "carbon ...
, automotive finishes, India ink
India ink (British English: Indian ink; also Chinese ink) is a simple black or coloured ink once widely used for writing and printing and now more commonly used for drawing and outlining, especially when inking comic books and comic strips. In ...
and laser printer
Laser printing is an electrostatic digital printing process. It produces high-quality text and graphics (and moderate-quality photographs) by repeatedly passing a laser beam back and forth over a Electric charge, negatively charged cylinder call ...
toner. Carbon black is also used as a filler in rubber products such as tyres and in plastic compounds. Activated charcoal
"Activated" is a song by English singer Cher Lloyd. It was released on 22 July 2016 through Vixen Records. The song was made available to stream exclusively on ''Rolling Stone'' a day before to release (on 21 July 2016).
Background
In an inter ...
is used as an absorbent and adsorbent
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a ...
in filter material in applications as diverse as gas masks, water purification, and kitchen extractor hood
A kitchen hood, exhaust hood, hood fan, extractor hood, or range hood is a device containing a mechanical fan that hangs above the stove or cooktop in the kitchen. It removes airborne grease, combustion products, fumes, smoke, heat, and steam fr ...
s, and in medicine to absorb toxins, poisons, or gases from the digestive system. Carbon is used in chemical reduction at high temperatures. Coke is used to reduce iron ore into iron (smelting). Case hardening
Case-hardening or carburization is the process of introducing carbon to the surface of a low-carbon iron, or more commonly a low-carbon steel object, in order to harden the surface.
Iron which has a carbon content greater than ~0.02% is known ...
of steel is achieved by heating finished steel components in carbon powder. Carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s of silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
, and titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
are among the hardest known materials, and are used as abrasives in cutting and grinding tools. Carbon compounds make up most of the materials used in clothing, such as natural and synthetic textiles and leather, and almost all of the interior surfaces in the built environment other than glass, stone, drywall, and metal.
Diamonds
Thediamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
industry falls into two categories: one dealing with gem-grade diamonds and the other, with industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets function dramatically differently. Unlike precious metal
Precious metals are rare, naturally occurring metallic chemical elements of high Value (economics), economic value. Precious metals, particularly the noble metals, are more corrosion resistant and less reactivity (chemistry), chemically reac ...
s such as gold or platinum, gem diamonds do not trade as a commodity. There is a substantial mark-up in the sale of diamonds, and there is not a very active market for resale of diamonds.
Industrial diamonds are valued mostly for their hardness and heat conductivity, with the gemological qualities of clarity and color being mostly irrelevant. About 80% of mined diamonds (equal to about 100 million carats or 20 tonnes annually) are unsuitable for use as gemstones and relegated for industrial use (known as ''bort
Bort, boart, or boort is an umbrella term used in the diamond industry to refer to shards of non- gem-grade/quality diamonds. In the manufacturing and heavy industries, "bort" is used to describe dark, imperfectly formed or crystallized diamond ...
)''. Synthetic diamond
A synthetic diamond or laboratory-grown diamond (LGD), also called a lab-grown, laboratory-created, man-made, artisan-created, artificial, or cultured diamond, is a diamond that is produced in a controlled technological process, in contrast to ...
s, invented in the 1950s, found almost immediate industrial applications; 3 billion carats (600 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s) of synthetic diamond is produced annually.
The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most of these applications do not require large diamonds; in fact, most diamonds of gem-quality except for their small size can be used industrially. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high-pressure experiments (see diamond anvil cell
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It permits the compression of a small (sub- millimeter-sized) piece of material to extreme pressures, typically up to around 1 ...
), high-performance bearings, and limited use in specialized windows. With the continuing advances in the production of synthetic diamonds, new applications are becoming feasible. Garnering much excitement is the possible use of diamond as a semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
suitable for microchips
An integrated circuit (IC), also known as a microchip or simply chip, is a set of electronic circuits, consisting of various electronic components (such as transistors, resistors, and capacitors) and their interconnections. These components a ...
, and because of its exceptional heat conductance property, as a heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is thermal management (electronics), ...
in electronics.
Precautions
 Pure carbon has extremely low toxicity to humans and can be handled safely in the form of graphite or charcoal. It is resistant to dissolution or chemical attack, even in the acidic contents of the digestive tract. Consequently, once it enters into the body's tissues it is likely to remain there indefinitely. Carbon black was probably one of the first pigments to be used for tattooing, and
Pure carbon has extremely low toxicity to humans and can be handled safely in the form of graphite or charcoal. It is resistant to dissolution or chemical attack, even in the acidic contents of the digestive tract. Consequently, once it enters into the body's tissues it is likely to remain there indefinitely. Carbon black was probably one of the first pigments to be used for tattooing, and Ötzi the Iceman
Ötzi, also called The Iceman, is the natural mummy of a man who lived between 3350 and 3105 BC. Ötzi's remains were discovered on 19 September 1991, in the Ötztal Alps (hence the nickname "Ötzi", ) at the Austria–Italy border. He ...
was found to have carbon tattoos that survived during his life and for 5200 years after his death. Inhalation of coal dust or soot (carbon black) in large quantities can be dangerous, irritating lung tissues and causing the congestive lung disease, coalworker's pneumoconiosis
Black lung disease (BLD), also known as coal workers' pneumoconiosis, or simply black lung, is an occupational disease, occupational type of pneumoconiosis caused by long-term inhalation and deposition of coal dust in the lungs and the consequent ...
. Diamond dust used as an abrasive can be harmful if ingested or inhaled. Microparticles of carbon are produced in diesel engine exhaust fumes, and may accumulate in the lungs. However, in these examples, the harm may result from contaminants (e.g., organic chemicals, heavy metals) rather than from the carbon itself.
Carbon may burn vigorously and brightly in the presence of air at high temperatures. Large accumulations of coal, which have remained inert for hundreds of millions of years in the absence of oxygen, may spontaneously combust when exposed to air in coal mine waste tips, ship cargo holds and coal bunkers, and storage dumps.
In nuclear applications where graphite is used as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely ...
, accumulation of Wigner energy followed by a sudden, spontaneous release may occur. Annealing to at least 250 °C can release the energy safely, although in the Windscale fire
The Windscale fire of 10 October 1957 was the worst nuclear accident in the United Kingdom's history, and one of the worst in the world, ranked in severity at level 5 out of 7 on the International Nuclear Event Scale. The fire was in Unit 1 of ...
the procedure went wrong, causing other reactor materials to combust.
The great variety of carbon compounds include such lethal poisons as tetrodotoxin
Tetrodotoxin (TTX) is a potent neurotoxin. Its name derives from Tetraodontiformes, an Order (biology), order that includes Tetraodontidae, pufferfish, porcupinefish, ocean sunfish, and triggerfish; several of these species carry the toxin. Alt ...
, the lectin
Lectins are carbohydrate-binding proteins that are highly specific for sugar Moiety (chemistry), groups that are part of other molecules, so cause agglutination (biology), agglutination of particular cells or precipitation of glycoconjugates an ...
ricin
Ricin ( ) is a lectin (a carbohydrate-binding protein) and a highly potent toxin produced in the seeds of the castor oil plant, ''Ricinus communis''. The median lethal dose (LD50) of ricin for mice is around 22 micrograms per kilogram of body ...
from seeds of the castor oil plant
''Ricinus communis'', the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, ''Ricinus'', and subtribe, Ricininae. The evolution of ca ...
''Ricinus communis
''Ricinus communis'', the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, ''Ricinus'', and subtribe, Ricininae. The evolution of ca ...
'', cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
(CN), and carbon monoxide; and such essentials to life as glucose and protein.
See also
*Carbon chauvinism
Carbon chauvinism is a neologism meant to disparage the assumption that the chemical processes of hypothetical extraterrestrial life must be constructed primarily from carbon (organic compounds) because as far as is known, carbon's chemical and ...
* Carbon detonation
Carbon detonation or carbon deflagration is the violent reignition of thermonuclear fusion in a white dwarf star that was previously slowly cooling. It involves a runaway thermonuclear process which spreads through the white dwarf in a matter of s ...
* Carbon footprint
A carbon footprint (or greenhouse gas footprint) is a calculated value or index that makes it possible to compare the total amount of greenhouse gases that an activity, product, company or country Greenhouse gas emissions, adds to the atmospher ...
* Carbon star
A carbon star (C-type star) is typically an asymptotic giant branch star, a luminous red giant, whose Stellar atmosphere, atmosphere contains more carbon than oxygen. The two elements combine in the upper layers of the star, forming carbon monox ...
* Carbon planet
A carbon planet is a hypothetical type of planet that contains more carbon than oxygen. Carbon is the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen.
Marc Kuchner and Sara Seager coined the term "carb ...
* Low-carbon economy
A low-carbon economy (LCE) is an economy which absorbs as much greenhouse gas as it emits. Greenhouse gas (GHG) emissions due to human activity are the dominant cause of observed climate change since the mid-20th century. There are many proven ...
* Timeline of carbon nanotubes
References
Bibliography
* * *External links
*Carbon
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Carbon on Britannica
(archived 18 June 2010)
(archived 9 November 2001)
Carbon—Super Stuff. Animation with sound and interactive 3D-models.
(archived 9 November 2012) {{Authority control Allotropes of carbon Chemical elements with hexagonal planar structure Chemical elements Native element minerals Polyatomic nonmetals Reactive nonmetals Reducing agents