|
Fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- and ellipsoid-like forms, cylinder (geometry), tubes, or other shapes. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball (association football), ball of association football. Nested closed fullerenes have been named bucky onions. Cylindrical fullerenes are also called carbon nanotubes or buckytubes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Buckminsterfullerene
Buckminsterfullerene is a type of fullerene with the formula . It has a cage-like fused-ring structure ( truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a football. Each of its 60 carbon atoms is bonded to its three neighbors. Buckminsterfullerene is a black solid that dissolves in hydrocarbon solvents to produce a violet solution. The substance was discovered in 1985 and has received intense study, although few real world applications have been found. Molecules of buckminsterfullerene (or of fullerenes in general) are commonly nicknamed buckyballs. Occurrence Buckminsterfullerene is the most common naturally occurring fullerene. Small quantities of it can be found in soot. It also exists in space. Neutral has been observed in planetary nebulae and several types of star. The ionised form, , has been identified in the interstellar medium, where it is the cause of several absorption features known as diffuse interstellar bands in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Allotropes Of Carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency (Tetravalence, tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, Carbon nanobud, nanobuds and Graphene nanoribbon, nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Atomic and diatomic carbon Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form a carbon arc under very low pressure. It is extrem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Nanotube Zigzag Povray Cropped
Carbon () is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
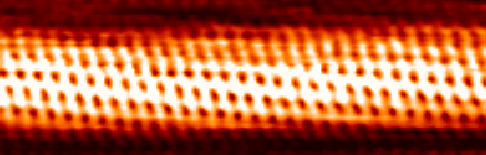
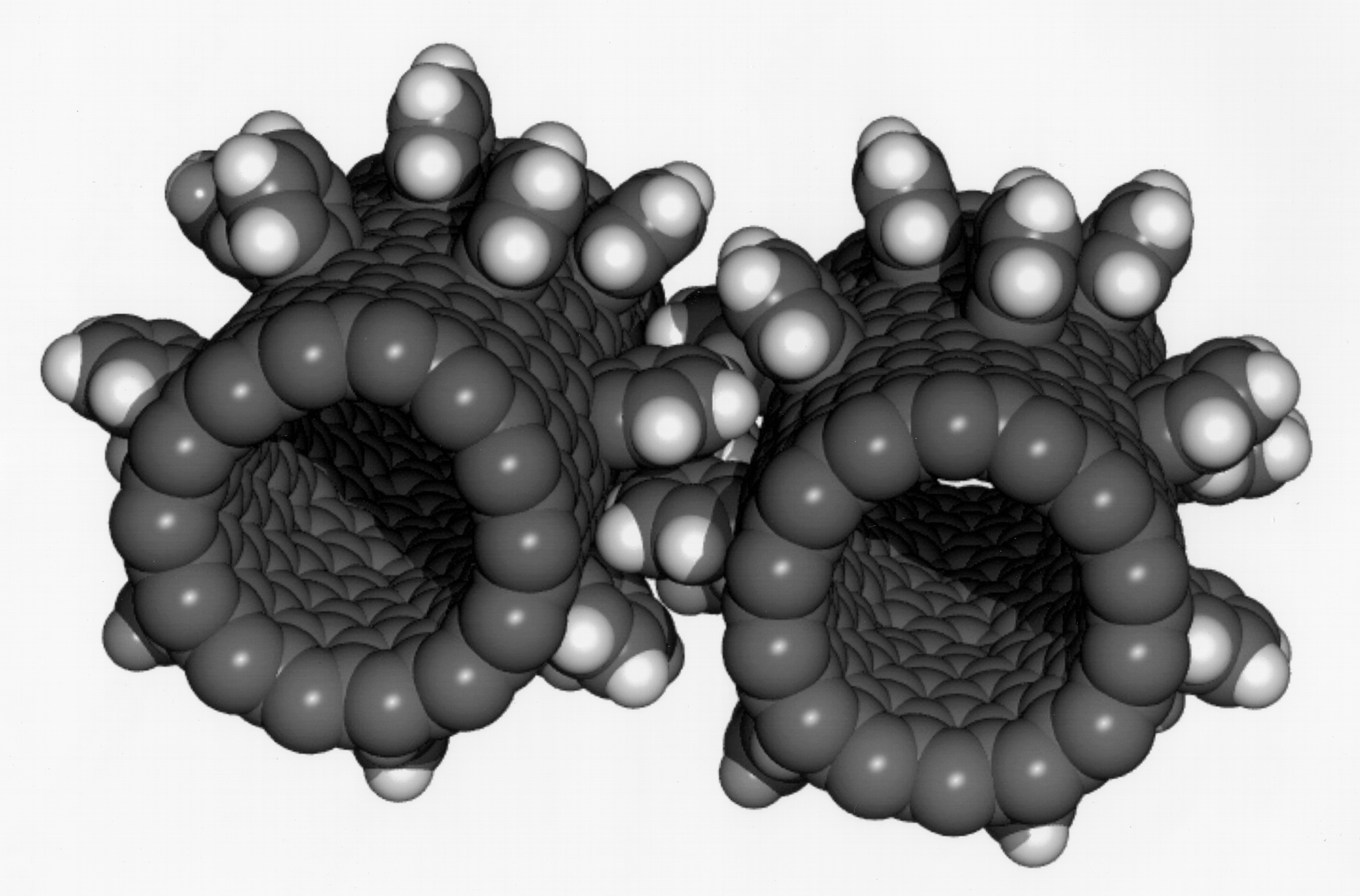
Carbon Nanotubes
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized: * ''Single-walled carbon nanotubes'' (''SWCNTs'') have diameters around 0.5–2.0 nanometer, nanometres, about 100,000 times smaller than the width of a human hair. They can be idealised as cutouts from a two-dimensional graphene sheet rolled up to form a hollow cylinder. * ''Multi-walled carbon nanotubes'' (''MWCNTs'') consist of nested single-wall carbon nanotubes in a nested, tube-in-tube structure. Double- and triple-walled carbon nanotubes are special cases of MWCNT. Carbon nanotubes can exhibit remarkable properties, such as exceptional tensile strength and thermal conductivity because of their nanostructure and bond strength, strength of the bonds between carbon atoms. Some SWCNT structures exhibit high electrical conductivity while others are semiconductors. In addition, ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Buckminster Fuller
Richard Buckminster Fuller (; July 12, 1895 – July 1, 1983) was an American architect, systems theorist, writer, designer, inventor, philosopher, and futurist. He styled his name as R. Buckminster Fuller in his writings, publishing more than 30 books and coining or popularizing such terms as " Spaceship Earth", "Dymaxion" (e.g., Dymaxion house, Dymaxion car, Dymaxion map), " ephemeralization", " synergetics", and "tensegrity". Fuller developed numerous inventions, mainly architectural designs, and popularized the widely known geodesic dome; carbon molecules known as fullerenes were later named by scientists for their structural and mathematical resemblance to geodesic spheres. He also served as the second World President of Mensa International from 1974 to 1983. Fuller was awarded 28 United States patents and many honorary doctorates. In 1960, he was awarded the Frank P. Brown Medal from The Franklin Institute. He was elected an honorary member of Phi Beta Kappa in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nanotechnology
Nanotechnology is the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing properties of matter. This definition of nanotechnology includes all types of research and technologies that deal with these special properties. It is common to see the plural form "nanotechnologies" as well as "nanoscale technologies" to refer to research and applications whose common trait is scale. An earlier understanding of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabricating macroscale products, now referred to as molecular nanotechnology. Nanotechnology defined by scale includes fields of science such as surface science, organic chemistry, molecular biology, semiconductor physics, energy storage, engineering, microfabrication, and molecular engineering. The associated rese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Corannulene
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20 H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is irculene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/ mol (42.7 kJ/mol) at −64 °C. Synthesis Several synthetic routes exist to corannulene. Flash vacuum pyrolysis techniques generally have lower chemical yields than solution-chemistry syntheses, but offer routes to more derivatives. Corannulene was first isolated in 1966 by multistep organic synthesis. In 1971, the synthesis and properties of corannulane were reported. A flash vacuum pyrolysis method followed in 1991. One synthesis based on soluti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Materials Science
Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries. The intellectual origins of materials science stem from the Age of Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Materials science still incorporates elements of physics, chemistry, and engineering. As such, the field was long considered by academic institutions as a sub-field of these related fields. Beginning in the 1940s, materials science began to be more widely recognized as a specific and distinct field of science and engineering, and major technical universities around the world created dedicated schools for its study. Materials scientists emphasize understanding how the history of a material (''processing'') influences its struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Eiji Osawa
is a former professor of computational chemistry, noted for his prediction of the C60 molecule in 1970. Osawa received his Master's of Engineering in chemistry from Kyoto University's Department of Industrial Chemistry and then became an engineer at Teijin Co., Ltd. In 1964, he returned to Kyoto University and earned a Doctorate of Engineering in chemistry under Professor M. Yoshida. After three years of postdoctoral work at the University of Wisconsin, Princeton University, and the State University of New York at Stony Brook, in 1970 he became an assistant professor at Hokkaido University. In 1990, Osawa became a full professor at Toyahashi University of Technology, where he retired in 2001. Upon his retirement, assisted by Futaba, Co., Ltd., headquartered in Chiba, Osawa started the research and development company Nano-Carbon Research Institute, Ltd. C60 molecule prediction The icosahedral C60H60 cage was mentioned in 1965 as a possible topological structure. Eiji Osawa pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
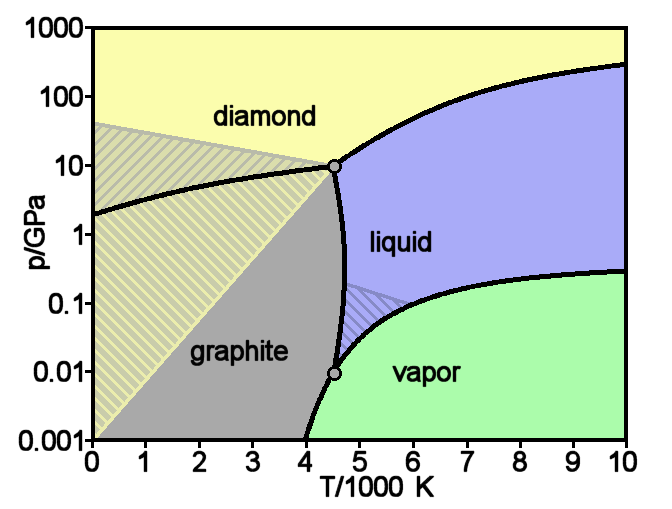
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
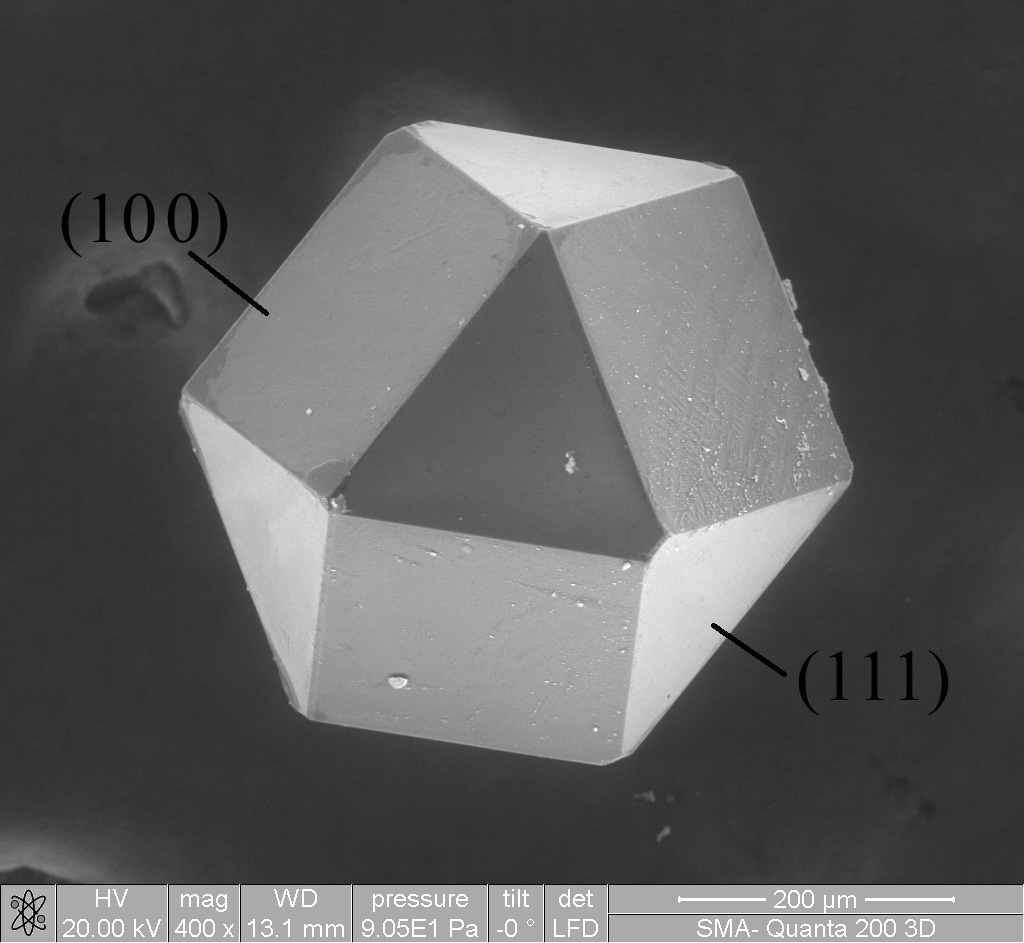
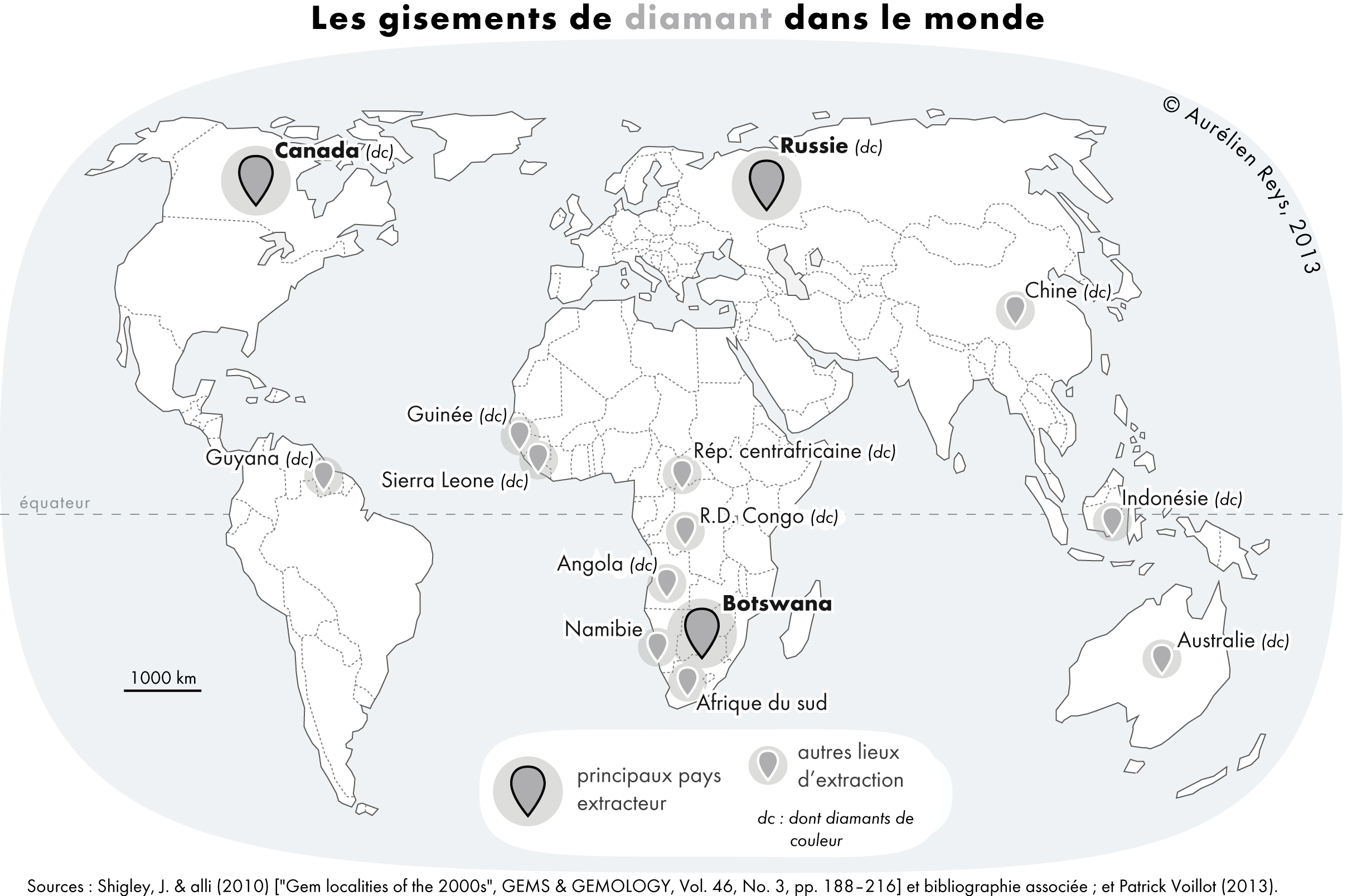
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) can color ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |