|
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Breath Test
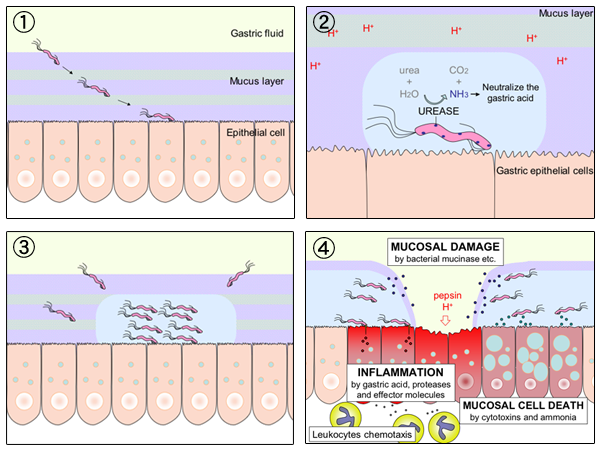
The urea breath test is a rapid diagnostic procedure used to identify infections by ''Helicobacter pylori'', a spiral bacterium implicated in gastritis, gastric ulcer, and peptic ulcer disease. It is based upon the ability of ''H. pylori'' to convert urea to ammonia and carbon dioxide. Urea breath tests are recommended in leading society guidelines as a preferred non-invasive choice for detecting ''H. pylori'' before and after treatment. Principles and mechanism Patients swallow urea labelled with an uncommon isotope, either radioactive carbon-14 (nowadays preferred in many countries) or non-radioactive carbon-13. In the subsequent 10–30 minutes, the detection of isotope-labelled carbon dioxide in exhaled breath indicates that the urea was split; this indicates that urease (the enzyme that ''H. pylori'' uses to metabolize urea to produce ammonia) is present in the stomach, and hence that ''H. pylori'' bacteria are present. For the two different forms of urea, different i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Isotopes
A medical isotope is an isotope used in medicine. The first uses of isotopes in medicine were in radiopharmaceuticals, and this is still the most common use. However more recently, separated stable isotopes have come into use. Radioactive isotopes Radioactive isotopes are used in medicine for both treatment and diagnostic scans. The most common isotope used in diagnostic scans is Technetium-99m, used in approximately 85% of all nuclear medicine diagnostic scans worldwide. It is used for diagnoses involving a large range of body parts and diseases such as cancers and neurological problems. Another well-known radioactive isotope used in medicine is Iodine-131, which is used as a radioactive label for some radiopharmaceutical therapies or the treatment of some types of thyroid cancer. Non-radioactive isotopes Examples of non-radioactive medical isotopes are: * Deuterium in deuterated drugs * Carbon-13 Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, C or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic matter is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (C), which makes up 99% of all carbon on Earth; carbon-13 (C), which makes up 1%; and carbon-14 (C), which occurs in trace amounts, making up about 1-1.5 atoms per 10 atoms of carbon in the atmosphere. C and C are both stable; C is unstable, with half-life years. Carbon-14 has a specific activity of 62.4 mCi/mmol (2.31 GBq/mmol), or 164.9 GBq/g. Carbon-14 decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13 Nuclear Magnetic Resonance
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR ( NMR) and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the isotope. The main carbon isotope, does not produce an NMR signal. Although ca. 1 mln. times less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds, primarily because 1H-decoupled 13C-NMR spectra are more simple, have a greater sensitivity to differences in the chemical structure, and, thus, are better suited for identifying molecules in complex mixtures. At the same time, such spectra lack quantitative information about the atomic ratios of different types of carbon nuclei, because nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope Labeling By Amino Acids In Cell Culture
Stable isotope labeling by/with amino acids in cell culture (SILAC) is a technique based on mass spectrometry that detects differences in protein abundance among samples using non-radioactive isotopic labeling. It is a popular method for quantitative proteomics. Procedure Two populations of cells are cultivated in cell culture. One of the cell populations is fed with growth medium containing normal amino acids. In contrast, the second population is fed with growth medium containing amino acids labeled with stable (non-radioactive) heavy isotopes. For example, the medium can contain arginine labeled with six carbon-13 atoms (13C) instead of the normal carbon-12 (12C). When the cells are growing in this medium, they incorporate the heavy arginine into all of their proteins. Thereafter, all peptides containing a single arginine are 6 Da heavier than their normal counterparts. Alternatively, uniform labeling with 13C or 15N can be used. Proteins from both cell populations are combined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
δ13C
In geochemistry, paleoclimatology, and paleoceanography ''δ''13C (pronounced "delta thirteen c") is an isotopic signature, a measure of the ratio of the two stable isotopes of carbon— 13C and 12C—reported in parts per thousand (per mil, ‰). The measure is also widely used in archaeology for the reconstruction of past diets, particularly to see if marine foods or certain types of plants were consumed. The definition is, in per mille: :\delta \ce = \left( \frac - 1 \right) \times 1000 where the standard is an established reference material. ''δ''13C varies in time as a function of productivity, the signature of the inorganic source, organic carbon burial, and vegetation type. Biological processes preferentially take up the lower mass isotope through kinetic fractionation. However some abiotic processes do the same. For example, methane from hydrothermal vents can be depleted by up to 50‰. Reference standard The standard established for carbon-13 work was the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons. History Before 1959, both the IUPAP and IUPAC used oxygen to define the mole; the chemists defining the mole as the number of atoms of oxygen which had mass 16 g, the physicists using a similar definition but with the oxygen-16 isotope only. The two organizations agreed in 1959–60 to define the mole as follows. ''Mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 12 gram of carbon 12; its symbol is "mol".'' This was adopted by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environmental Isotopes
The environmental isotopes are a subset of isotopes, both Stable isotope ratio, stable and Radioactive isotopes, radioactive, which are the object of isotope geochemistry. They are primarily used as tracers to see how things move around within the ocean-atmosphere system, within terrestrial biomes, within the Earth's surface, and between these broad domains. Isotope geochemistry Chemical elements are defined by their number of protons, but the atomic mass, mass of the atom is determined by the number of protons and neutrons in the nucleus. Isotopes are atoms that are of a specific element, but have different numbers of neutrons and thus different mass numbers. The ratio between isotopes of an element varies slightly in the world, so in order to study isotopic ratio changes across the world, changes in isotope ratios are defined as deviations from a standard, multiplied by 1000. This unit is a "per mil". As a convention, the ratio is of the heavier isotope to the lower isotope. \d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Ratio Mass Spectrometry
Isotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample. This technique has two different applications in the earth and environmental sciences. The analysis of 'Stable isotope ratio, stable isotopes' is normally concerned with measuring isotopic variations arising from mass-dependent isotopic fractionation in natural systems. On the other hand, radiogenic isotope analysis involves measuring the abundances of decay-products of natural radioactivity, and is used in most long-lived radiometric dating methods. Introduction The isotope-ratio mass spectrometer (IRMS) allows the precise measurement of mixtures of naturally occurring isotopes. Most instruments used for precise determination of isotope ratios are of the magnetic sector instrument, sector type. This type of analyzer is superior to the Quadrupole mass analyzer, quadrupole type in this field ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helicobacter Pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, Flagellum#bacterial, flagellated, Bacterial cellular morphologies#Helical, helical bacterium. Mutants can have a rod or curved rod shape that exhibits less virulence. Its Helix, helical body (from which the genus name ''Helicobacter'' derives) is thought to have evolved to penetrate the gastric mucosa, mucous lining of the stomach, helped by its flagella, and thereby establish infection. While many earlier reports of an association between bacteria and the ulcers had existed, such as the works of John Lykoudis, it was only in 1983 when the bacterium was formally described for the first time in the English-language Western literature as the causal agent of peptic ulcer, gastric ulcers by Australian physician-scientists Barry Marshall and Robin Warren. In 2005, the pair was awarded the Nobel Prize in Physiology or Medicine for their discovery. Infection of the stomach with ''H. pylori'' doe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |