
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.
[International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.php][ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology] Refrigeration can be considered an artificial, or human-made,
cooling
Cooling is removal of heat, usually resulting in a lower temperature and/or phase change. Temperature lowering achieved by any other means may also be called cooling.ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/as ...
method.
Refrigeration refers to the process by which energy, in the form of heat, is removed from a low-temperature medium and transferred to a high-temperature medium. This work of energy transfer is traditionally driven by
mechanical
Mechanical may refer to:
Machine
* Machine (mechanical), a system of mechanisms that shape the actuator input to achieve a specific application of output forces and movement
* Mechanical calculator, a device used to perform the basic operations of ...
means, but can also be driven by heat,
magnetism
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particles ...
,
electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
,
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The fir ...
, or other means. Refrigeration has many applications, including household
refrigerators, industrial
freezer
A refrigerator, colloquially fridge, is a commercial and home appliance consisting of a thermally insulated compartment and a heat pump (mechanical, electronic or chemical) that transfers heat from its inside to its external environment so th ...
s,
cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
, and
air conditioning
Air conditioning, often abbreviated as A/C or AC, is the process of removing heat from an enclosed space to achieve a more comfortable interior environment (sometimes referred to as 'comfort cooling') and in some cases also strictly controlling ...
.
Heat pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing ...
s may use the heat output of the refrigeration process, and also may be designed to be reversible, but are otherwise similar to air conditioning units.
Refrigeration has had a large impact on industry, lifestyle, agriculture, and settlement patterns. The idea of preserving food dates back to the
ancient Roman empire
The Roman Empire ( la, Imperium Romanum ; grc-gre, Βασιλεία τῶν Ῥωμαίων, Basileía tôn Rhōmaíōn) was the post-Republican period of ancient Rome. As a polity, it included large territorial holdings around the Mediterr ...
. Mechanical refrigeration has rapidly evolved in the last century, from
ice harvesting
Ice cutting is a winter task of collecting surface ice from lakes and rivers for storage in ice houses and use or sale as a cooling method. Rare today, it was common (see ice trade) before the era of widespread mechanical refrigeration and air ...
to
temperature-controlled rail cars. The introduction of refrigerated rail cars contributed to the westward expansion of the United States, allowing settlement in areas that were not on main transport channels such as rivers, harbors, or valley trails. Settlements were also developing in infertile parts of the country, filled with newly discovered natural resources.
These new settlement patterns sparked the building of large cities which are able to thrive in areas that were otherwise thought to be inhospitable, such as
Houston
Houston (; ) is the most populous city in Texas, the most populous city in the Southern United States, the fourth-most populous city in the United States, and the sixth-most populous city in North America, with a population of 2,304,580 in ...
, Texas, and
Las Vegas
Las Vegas (; Spanish for "The Meadows"), often known simply as Vegas, is the 25th-most populous city in the United States, the most populous city in the state of Nevada, and the county seat of Clark County. The city anchors the Las Vegas ...
, Nevada. In most developed countries, cities are heavily dependent upon refrigeration in
supermarket
A supermarket is a self-service Retail#Types of outlets, shop offering a wide variety of food, Drink, beverages and Household goods, household products, organized into sections. This kind of store is larger and has a wider selection than earli ...
s in order to obtain their food for daily consumption. The increase in food sources has led to a larger concentration of agricultural sales coming from a smaller percentage of farms. Farms today have a much larger output per person in comparison to the late 1800s. This has resulted in new food sources available to entire populations, which has had a large impact on the nutrition of society.
History
Earliest forms of cooling
The seasonal harvesting of snow and ice is an ancient practice estimated to have begun earlier than 1000 BC.
A Chinese collection of lyrics from this time period known as the ''
Shijing
The ''Classic of Poetry'', also ''Shijing'' or ''Shih-ching'', translated variously as the ''Book of Songs'', ''Book of Odes'', or simply known as the ''Odes'' or ''Poetry'' (; ''Shī''), is the oldest existing collection of Chinese poetry, co ...
'', describes religious ceremonies for filling and emptying ice cellars. However, little is known about the construction of these ice cellars or the purpose of the ice. The next ancient society to record the harvesting of ice may have been the Jews in the book of Proverbs, which reads, "As the cold of snow in the time of harvest, so is a faithful messenger to them who sent him." Historians have interpreted this to mean that the Jews used ice to cool beverages rather than to preserve food. Other ancient cultures such as the Greeks and the Romans dug large snow pits insulated with grass, chaff, or branches of trees as cold storage. Like the Jews, the Greeks and Romans did not use ice and snow to preserve food, but primarily as a means to cool beverages. Egyptians cooled water by evaporation in shallow earthen jars on the roofs of their houses at night. The ancient people of India used this same concept to produce ice. The Persians stored ice in a pit called a
Yakhchal and may have been the first group of people to use cold storage to preserve food. In the Australian outback before a reliable electricity supply was available many farmers used a
Coolgardie safe
The Coolgardie safe is a low-tech food storage unit, using evaporative cooling to prolong the life of whatever edibles are kept in it. It applies the basic principle of heat transfer which occurs during evaporation of water (see latent heat and ...
, consisting of a room with
hessian (burlap) curtains hanging from the ceiling soaked in water. The water would evaporate and thereby cool the room, allowing many perishables such as fruit, butter, and cured meats to be kept.
Ice harvesting

Before 1830, few Americans used ice to refrigerate foods due to a lack of ice-storehouses and iceboxes. As these two things became more widely available, individuals used axes and saws to
harvest ice for their storehouses. This method proved to be difficult, dangerous, and certainly did not resemble anything that could be duplicated on a commercial scale.
Despite the difficulties of harvesting ice, Frederic Tudor thought that he could capitalize on this new commodity by harvesting ice in New England and shipping it to the Caribbean islands as well as the southern states. In the beginning, Tudor lost thousands of dollars, but eventually turned a profit as he constructed icehouses in Charleston, Virginia and in the Cuban port town of Havana. These icehouses as well as better insulated ships helped reduce ice wastage from 66% to 8%. This efficiency gain influenced Tudor to expand his ice market to other towns with icehouses such as New Orleans and Savannah. This ice market further expanded as harvesting ice became faster and cheaper after one of Tudor's suppliers, Nathaniel Wyeth, invented a horse-drawn ice cutter in 1825. This invention as well as Tudor's success inspired others to get involved in the
ice trade
The ice trade, also known as the frozen water trade, was a 19th-century and early-20th-century industry, centering on the east coast of the United States and Norway, involving the large-scale harvesting, transport and sale of natural ice, an ...
and the ice industry grew.
Ice became a mass-market commodity by the early 1830s with the price of ice dropping from six cents per pound to a half of a cent per pound. In New York City, ice consumption increased from 12,000 tons in 1843 to 100,000 tons in 1856. Boston's consumption leapt from 6,000 tons to 85,000 tons during that same period. Ice harvesting created a "cooling culture" as majority of people used ice and iceboxes to store their dairy products, fish, meat, and even fruits and vegetables. These early cold storage practices paved the way for many Americans to accept the refrigeration technology that would soon take over the country.
Refrigeration research
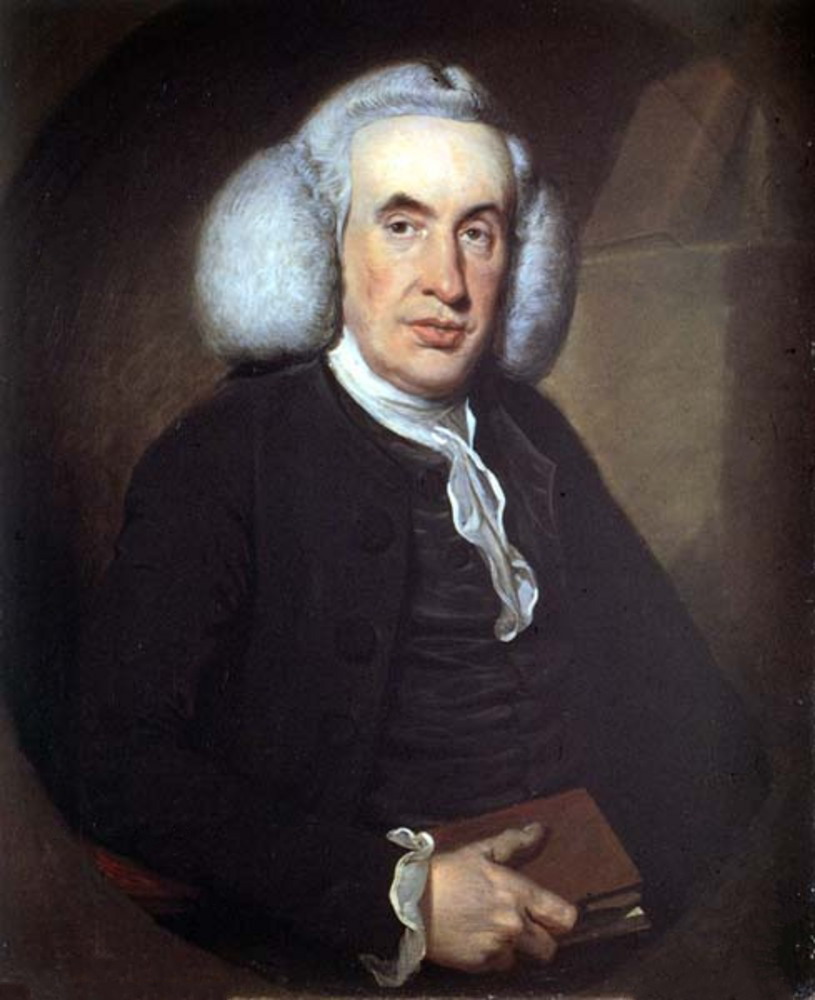
The history of artificial refrigeration began when Scottish professor
William Cullen
William Cullen FRS FRSE FRCPE FPSG (; 15 April 17105 February 1790) was a Scottish physician, chemist and agriculturalist, and professor at the Edinburgh Medical School. Cullen was a central figure in the Scottish Enlightenment: He was ...
designed a small refrigerating machine in 1755. Cullen used a pump to create a partial
vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often dis ...
over a container of
diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
, which then
boiled
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. T ...
, absorbing
heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is al ...
from the surrounding air. The experiment even created a small amount of ice, but had no practical application at that time.
In 1758,
Benjamin Franklin
Benjamin Franklin ( April 17, 1790) was an American polymath who was active as a writer, scientist, inventor, statesman, diplomat, printer, publisher, and political philosopher. Encyclopædia Britannica, Wood, 2021 Among the leading inte ...
and
John Hadley
John Hadley (16 April 1682 – 14 February 1744) was an English mathematician, and laid claim to the invention of the octant, two years after Thomas Godfrey claimed the same.
Biography
He was born in Bloomsbury, London the eldest son of ...
, professor of chemistry, collaborated on a project investigating the principle of evaporation as a means to rapidly cool an object at
Cambridge University
, mottoeng = Literal: From here, light and sacred draughts.
Non literal: From this place, we gain enlightenment and precious knowledge.
, established =
, other_name = The Chancellor, Masters and Schola ...
,
England
England is a country that is part of the United Kingdom. It shares land borders with Wales to its west and Scotland to its north. The Irish Sea lies northwest and the Celtic Sea to the southwest. It is separated from continental Europe b ...
. They confirmed that the evaporation of highly volatile liquids, such as alcohol and ether, could be used to drive down the temperature of an object past the freezing point of water. They conducted their experiment with the bulb of a mercury thermometer as their object and with a bellows used to quicken the evaporation; they lowered the temperature of the thermometer bulb down to , while the ambient temperature was . They noted that soon after they passed the freezing point of water , a thin film of ice formed on the surface of the thermometer's bulb and that the ice mass was about a thick when they stopped the experiment upon reaching . Franklin wrote, "From this experiment, one may see the possibility of freezing a man to death on a warm summer's day". In 1805, American inventor
Oliver Evans
Oliver Evans (September 13, 1755 – April 15, 1819) was an American inventor, engineer and businessman born in rural Delaware and later rooted commercially in Philadelphia. He was one of the first Americans building steam engines and an advoca ...
described a closed
vapor-compression refrigeration
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings ...
cycle for the production of ice by ether under vacuum.
In 1820, the English scientist
Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inducti ...
liquefied
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
and other gases by using high pressures and low temperatures, and in 1834, an American expatriate to Great Britain,
Jacob Perkins
Jacob Perkins (9 July 1766 – 30 July 1849) was an American inventor, mechanical engineer and physicist. Born in Newburyport, Massachusetts, Perkins was apprenticed to a goldsmith. He soon made himself known with a variety of useful mechanical i ...
, built the first working vapor-compression refrigeration system in the world. It was a closed-cycle that could operate continuously, as he described in his patent:
:I am enabled to use volatile fluids for the purpose of producing the cooling or freezing of fluids, and yet at the same time constantly condensing such volatile fluids, and bringing them again into operation without waste.
His prototype system worked although it did not succeed commercially.
In 1842, a similar attempt was made by American physician,
John Gorrie
John B. Gorrie (October 3, 1803 – June 29, 1855) was a Nevisian-born American physician and scientist, credited as the inventor of mechanical refrigeration.
Early life
Born on the Island of Nevis in the Leeward Islands of the West Indies t ...
, who built a working prototype, but it was a commercial failure. Like many of the medical experts during this time, Gorrie thought too much exposure to tropical heat led to mental and physical degeneration, as well as the spread of diseases such as malaria. He conceived the idea of using his refrigeration system to cool the air for comfort in homes and hospitals to prevent disease. American engineer
Alexander Twining took out a British patent in 1850 for a vapour compression system that used ether.
The first practical vapour-compression refrigeration system was built by
James Harrison, a British journalist who had emigrated to
Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
. His 1856 patent was for a vapour-compression system using ether, alcohol, or ammonia. He built a mechanical ice-making machine in 1851 on the banks of the Barwon River at Rocky Point in
Geelong
Geelong ( ) (Wathawurrung: ''Djilang''/''Djalang'') is a port city in the southeastern Australian state of Victoria, located at the eastern end of Corio Bay (the smaller western portion of Port Phillip Bay) and the left bank of Barwon River, ...
,
Victoria
Victoria most commonly refers to:
* Victoria (Australia), a state of the Commonwealth of Australia
* Victoria, British Columbia, provincial capital of British Columbia, Canada
* Victoria (mythology), Roman goddess of Victory
* Victoria, Seychelle ...
, and his first commercial ice-making machine followed in 1854. Harrison also introduced commercial vapour-compression refrigeration to breweries and meat-packing houses, and by 1861, a dozen of his systems were in operation. He later entered the debate of how to compete against the American advantage of unrefrigerated
beef
Beef is the culinary name for meat from cattle (''Bos taurus'').
In prehistoric times, humankind hunted aurochs and later domesticated them. Since that time, numerous breeds of cattle have been bred specifically for the quality or quantit ...
sales to the
United Kingdom
The United Kingdom of Great Britain and Northern Ireland, commonly known as the United Kingdom (UK) or Britain, is a country in Europe, off the north-western coast of the continental mainland. It comprises England, Scotland, Wales and North ...
. In 1873 he prepared the sailing ship ''Norfolk'' for an experimental beef shipment to the United Kingdom, which used a cold room system instead of a refrigeration system. The venture was a failure as the ice was consumed faster than expected.
The first
gas absorption
Sorption is a physical and chemical process by which one substance becomes attached to another. Specific cases of sorption are treated in the following articles:
; Absorption: "the incorporation of a substance in one state into another of a d ...
refrigeration system using gaseous ammonia dissolved in water (referred to as "aqua ammonia") was developed by
Ferdinand Carré
Ferdinand Philippe Edouard Carré (11 March 1824 – 11 January 1900) was a French engineer, born at Moislains (Somme) on 11 March 1824. Carré is best known as the inventor of refrigeration equipment used to produce ice. He died on 11 January 19 ...
of France in 1859 and patented in 1860.
Carl von Linde
Carl Paul Gottfried von Linde (11 June 1842 – 16 November 1934) was a German scientist, engineer, and businessman. He discovered a refrigeration cycle and invented the first industrial-scale air separation and gas liquefaction processes, whi ...
, an engineer specializing in
steam locomotive
A steam locomotive is a locomotive that provides the force to move itself and other vehicles by means of the expansion of steam. It is fuelled by burning combustible material (usually coal, oil or, rarely, wood) to heat water in the locomot ...
s and professor of engineering at the
Technological University of Munich in Germany, began researching refrigeration in the 1860s and 1870s in response to demand from brewers for a technology that would allow year-round, large-scale production of
lager
Lager () is beer which has been brewed and conditioned at low temperature. Lagers can be pale, amber, or dark. Pale lager is the most widely consumed and commercially available style of beer. The term "lager" comes from the German for "storag ...
; he patented an improved method of liquefying gases in 1876. His new process made possible using gases such as
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
,
sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
(SO
2) and
methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industria ...
(CH
3Cl) as refrigerants and they were widely used for that purpose until the late 1920s.
Thaddeus Lowe
Thaddeus Sobieski Constantine Lowe (August 20, 1832 – January 16, 1913), also known as Professor T. S. C. Lowe, was an American Civil War aeronaut, scientist and inventor, mostly self-educated in the fields of chemistry, meteorology, and a ...
, an American balloonist, held several patents on ice-making machines. His "Compression Ice Machine" would revolutionize the cold-storage industry. In 1869, other investors and he purchased an old steamship onto which they loaded one of Lowe's refrigeration units and began shipping fresh fruit from New York to the Gulf Coast area, and fresh meat from Galveston, Texas back to New York, but because of Lowe's lack of knowledge about shipping, the business was a costly failure.
Commercial use

In 1842,
John Gorrie
John B. Gorrie (October 3, 1803 – June 29, 1855) was a Nevisian-born American physician and scientist, credited as the inventor of mechanical refrigeration.
Early life
Born on the Island of Nevis in the Leeward Islands of the West Indies t ...
created a system capable of refrigerating water to produce ice. Although it was a commercial failure, it inspired scientists and inventors around the world. France's Ferdinand Carre was one of the inspired and he created an ice producing system that was simpler and smaller than that of Gorrie. During the Civil War, cities such as New Orleans could no longer get ice from New England via the coastal ice trade. Carre's refrigeration system became the solution to New Orleans ice problems and, by 1865, the city had three of Carre's machines. In 1867, in San Antonio, Texas, a French immigrant named Andrew Muhl built an ice-making machine to help service the expanding beef industry before moving it to Waco in 1871. In 1873, the patent for this machine was contracted by the Columbus Iron Works, a company acquired by the W.C. Bradley Co., which went on to produce the first commercial ice-makers in the US.
By the 1870s, breweries had become the largest users of harvested ice. Though the ice-harvesting industry had grown immensely by the turn of the 20th century, pollution and sewage had begun to creep into natural ice, making it a problem in the metropolitan suburbs. Eventually, breweries began to complain of tainted ice. Public concern for the purity of water, from which ice was formed, began to increase in the early 1900s with the rise of germ theory. Numerous media outlets published articles connecting diseases such as typhoid fever with natural ice consumption. This caused ice harvesting to become illegal in certain areas of the country. All of these scenarios increased the demands for modern refrigeration and manufactured ice. Ice producing machines like that of Carre's and Muhl's were looked to as means of producing ice to meet the needs of grocers, farmers, and food shippers.
Refrigerated railroad cars were introduced in the US in the 1840s for short-run transport of dairy products, but these used harvested ice to maintain a cool temperature.

The new refrigerating technology first met with widespread industrial use as a means to freeze meat supplies for transport by sea in
reefer ship
A reefer ship is a refrigerated cargo ship typically used to transport perishable cargo, which require temperature-controlled handling, such as fruits, meat, vegetables, dairy products, and similar items.
Description
''Types of reefers:'' Re ...
s from the British
Dominion
The term ''Dominion'' is used to refer to one of several self-governing nations of the British Empire.
"Dominion status" was first accorded to Canada, Australia, New Zealand, Newfoundland, South Africa, and the Irish Free State at the 192 ...
s and other countries to the
British Isles
The British Isles are a group of islands in the North Atlantic Ocean off the north-western coast of continental Europe, consisting of the islands of Great Britain, Ireland, the Isle of Man, the Inner and Outer Hebrides, the Northern Isles, ...
. Although not actually the first to achieve successful transportation of frozen goods overseas (the ''Strathleven'' had arrived at the London docks on 2 February 1880 with a cargo of frozen beef, mutton and butter from Sydney and Melbourne ), the breakthrough is often attributed to
William Soltau Davidson
William Soltau Davidson (15 June 1846 – 17 July 1924) was the New Zealand pioneer of refrigerated shipping.
Early life
Son of Frances Pillans and bank manager David Davidson, William Davidson was born in Montreal, Canada. He attended the Edi ...
, an entrepreneur who had emigrated to
New Zealand
New Zealand ( mi, Aotearoa ) is an island country in the southwestern Pacific Ocean. It consists of two main landmasses—the North Island () and the South Island ()—and over 700 smaller islands. It is the sixth-largest island count ...
. Davidson thought that Britain's rising population and meat demand could mitigate the slump in world
wool
Wool is the textile fibre obtained from sheep and other mammals, especially goats, rabbits, and camelids. The term may also refer to inorganic materials, such as mineral wool and glass wool, that have properties similar to animal wool.
As ...
markets that was heavily affecting New Zealand. After extensive research, he commissioned the
''Dunedin'' to be refitted with a compression refrigeration unit for meat shipment in 1881. On February 15, 1882, the ''Dunedin'' sailed for London with what was to be the first commercially successful refrigerated shipping voyage, and the foundation of the refrigerated
meat industry
The meat industry are the people and companies engaged in modern industrialized livestock agriculture for the production, packing, preservation and marketing of meat (in contrast to dairy products, wool, etc.). In economics, the meat industry is ...
.
''
The Times
''The Times'' is a British daily national newspaper based in London. It began in 1785 under the title ''The Daily Universal Register'', adopting its current name on 1 January 1788. ''The Times'' and its sister paper ''The Sunday Times'' (fou ...
'' commented "Today we have to record such a triumph over physical difficulties, as would have been incredible, even unimaginable, a very few days ago...". The ''
Marlborough
Marlborough may refer to:
Places United Kingdom
* Marlborough, Wiltshire, England
** Marlborough College, public school
* Marlborough School, Woodstock in Oxfordshire, England
* The Marlborough Science Academy in Hertfordshire, England
Austral ...
''—sister ship to the ''Dunedin'' – was immediately converted and joined the trade the following year, along with the rival
New Zealand Shipping Company
The New Zealand Shipping Company (NZSC) was a shipping company whose ships ran passenger and cargo services between Great Britain and New Zealand between 1873 and 1973.
A group of Christchurch businessmen founded the company in 1873, similar ...
vessel ''Mataurua'', while the German Steamer ''Marsala'' began carrying frozen New Zealand lamb in December 1882. Within five years, 172 shipments of frozen meat were sent from New Zealand to the United Kingdom, of which only 9 had significant amounts of meat condemned. Refrigerated shipping also led to a broader meat and dairy boom in
Australasia
Australasia is a region that comprises Australia, New Zealand and some neighbouring islands in the Pacific Ocean. The term is used in a number of different contexts, including geopolitically, physiogeographically, philologically, and ecologica ...
and South America.
J & E Hall
J & E Hall is an English manufacturer of refrigeration equipment (today part of the Daikin group). It was originally established as an iron works in Dartford, Kent in 1785, with products including papermaking machines, steam engines and gun carriag ...
of
Dartford
Dartford is the principal town in the Borough of Dartford, Kent, England. It is located south-east of Central London and
is situated adjacent to the London Borough of Bexley to its west. To its north, across the Thames estuary, is Thurrock in ...
, England outfitted the ''SS Selembria'' with a vapor compression system to bring 30,000 carcasses of
mutton
Lamb, hogget, and mutton, generically sheep meat, are the meat of domestic sheep, ''Ovis aries''. A sheep in its first year is a lamb and its meat is also lamb. The meat from sheep in their second year is hogget. Older sheep meat is mutton. Gen ...
from the
Falkland Islands
The Falkland Islands (; es, Islas Malvinas, link=no ) is an archipelago in the South Atlantic Ocean on the Patagonian Shelf. The principal islands are about east of South America's southern Patagonian coast and about from Cape Dubouzet ...
in 1886. In the years ahead, the industry rapidly expanded to Australia, Argentina and the United States.
By the 1890s, refrigeration played a vital role in the distribution of food. The meat-packing industry relied heavily on natural ice in the 1880s and continued to rely on manufactured ice as those technologies became available. By 1900, the meat-packing houses of Chicago had adopted ammonia-cycle commercial refrigeration. By 1914, almost every location used artificial refrigeration. The
major meat packers, Armour, Swift, and Wilson, had purchased the most expensive units which they installed on train cars and in branch houses and storage facilities in the more remote distribution areas.
By the middle of the 20th century, refrigeration units were designed for installation on trucks or lorries. Refrigerated vehicles are used to transport perishable goods, such as frozen foods, fruit and vegetables, and temperature-sensitive chemicals. Most modern refrigerators keep the temperature between –40 and –20 °C, and have a maximum payload of around 24,000 kg gross weight (in Europe).
Although commercial refrigeration quickly progressed, it had limitations that prevented it from moving into the household. First, most refrigerators were far too large. Some of the commercial units being used in 1910 weighed between five and two hundred tons. Second, commercial refrigerators were expensive to produce, purchase, and maintain. Lastly, these refrigerators were unsafe. It was not uncommon for commercial refrigerators to catch fire, explode, or leak toxic gases. Refrigeration did not become a household technology until these three challenges were overcome.
Home and consumer use


During the early 1800s, consumers preserved their food by storing food and ice purchased from ice harvesters in iceboxes. In 1803, Thomas Moore patented a metal-lined butter-storage tub which became the prototype for most iceboxes. These iceboxes were used until nearly 1910 and the technology did not progress. In fact, consumers that used the icebox in 1910 faced the same challenge of a moldy and stinky icebox that consumers had in the early 1800s.
General Electric (GE) was one of the first companies to overcome these challenges. In 1911, GE released a household refrigeration unit that was powered by gas. The use of gas eliminated the need for an electric compressor motor and decreased the size of the refrigerator. However, electric companies that were customers of GE did not benefit from a gas-powered unit. Thus, GE invested in developing an electric model. In 1927, GE released the Monitor Top, the first refrigerator to run on electricity.
In 1930, Frigidaire, one of GE's main competitors, synthesized
Freon
Freon ( ) is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol prope ...
. With the invention of synthetic refrigerants based mostly on a chlorofluorocarbon (CFC) chemical, safer refrigerators were possible for home and consumer use. Freon led to the development of smaller, lighter, and cheaper refrigerators. The average price of a refrigerator dropped from $275 to $154 with the synthesis of Freon. This lower price allowed ownership of refrigerators in American households to exceed 50% by 1940. Freon is a trademark of the DuPont Corporation and refers to these CFCs, and later hydro chlorofluorocarbon (HCFC) and hydro fluorocarbon (HFC), refrigerants developed in the late 1920s. These refrigerants were considered — at the time — to be less harmful than the commonly-used refrigerants of the time, including methyl formate, ammonia, methyl chloride, and sulfur dioxide. The intent was to provide refrigeration equipment for home use without danger. These CFC refrigerants answered that need. In the 1970s, though, the compounds were found to be reacting with atmospheric ozone, an important protection against solar ultraviolet radiation, and their use as a refrigerant worldwide was curtailed in the
Montreal Protocol
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion
Ozone depletion consists of two related events observed sinc ...
of 1987.
Impact on settlement patterns
In the last century, refrigeration allowed new settlement patterns to emerge. This new technology has allowed for new areas to be settled that are not on a natural channel of transport such as a river, valley trail or harbor that may have otherwise not been settled. Refrigeration has given opportunities to early settlers to expand westward and into rural areas that were unpopulated. These new settlers with rich and untapped soil saw opportunity to profit by sending raw goods to the eastern cities and states. In the 20th century, refrigeration has made "Galactic Cities" such as Dallas, Phoenix and Los Angeles possible.
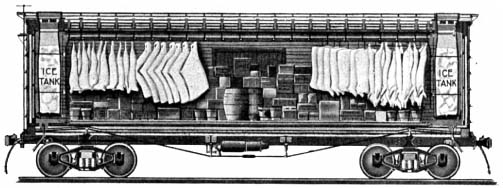
Refrigerated rail cars
The refrigerated rail car (
refrigerated van
A refrigerated van (also called a refrigerated wagon) is a railway goods wagon with cooling equipment. Today they are designated by the International Union of Railways (UIC) as Class I.
History
The first wagons were cooled with ice that had ...
or
refrigerator car
A refrigerator car (or "reefer") is a refrigerated boxcar (U.S.), a piece of railroad rolling stock designed to carry perishable freight at specific temperatures. Refrigerator cars differ from simple insulated boxcars and ventilated boxcars (co ...
), along with the dense railroad network, became an exceedingly important link between the marketplace and the farm allowing for a national opportunity rather than a just a regional one. Before the invention of the refrigerated rail car, it was impossible to ship perishable food products long distances. The beef packing industry made the first demand push for refrigeration cars. The railroad companies were slow to adopt this new invention because of their heavy investments in cattle cars, stockyards, and feedlots. Refrigeration cars were also complex and costly compared to other rail cars, which also slowed the adoption of the refrigerated rail car. After the slow adoption of the refrigerated car, the beef packing industry dominated the refrigerated rail car business with their ability to control ice plants and the setting of icing fees. The United States Department of Agriculture estimated that, in 1916, over sixty-nine percent of the cattle killed in the country was done in plants involved in interstate trade. The same companies that were also involved in the meat trade later implemented refrigerated transport to include vegetables and fruit. The meat packing companies had much of the expensive machinery, such as refrigerated cars, and cold storage facilities that allowed for them to effectively distribute all types of perishable goods. During World War I, a national refrigerator car pool was established by the United States Administration to deal with problem of idle cars and was later continued after the war. The idle car problem was the problem of refrigeration cars sitting pointlessly in between seasonal harvests. This meant that very expensive cars sat in rail yards for a good portion of the year while making no revenue for the car's owner. The car pool was a system where cars were distributed to areas as crops matured ensuring maximum use of the cars. Refrigerated rail cars moved eastward from vineyards, orchards, fields, and gardens in western states to satisfy Americas consuming market in the east. The refrigerated car made it possible to transport perishable crops hundreds and even thousands of kilometres or miles. The most noticeable effect the car gave was a regional specialization of vegetables and fruits. The refrigeration rail car was widely used for the transportation of perishable goods up until the 1950s. By the 1960s, the nation's interstate highway system was adequately complete allowing for trucks to carry the majority of the perishable food loads and to push out the old system of the refrigerated rail cars.
Expansion west and into rural areas
The widespread use of refrigeration allowed for a vast amount of new agricultural opportunities to open up in the United States. New markets emerged throughout the United States in areas that were previously uninhabited and far-removed from heavily populated areas. New agricultural opportunity presented itself in areas that were considered rural, such as states in the south and in the west. Shipments on a large scale from the south and California were both made around the same time, although natural ice was used from the Sierras in California rather than manufactured ice in the south. Refrigeration allowed for many areas to specialize in the growing of specific fruits. California specialized in several fruits, grapes, peaches, pears, plums, and apples, while Georgia became famous for specifically its peaches. In California, the acceptance of the refrigerated rail cars led to an increase of car loads from 4,500 carloads in 1895 to between 8,000 and 10,000 carloads in 1905. The Gulf States, Arkansas, Missouri and Tennessee entered into strawberry production on a large-scale while Mississippi became the center of the
tomato industry
The tomato is the edible berry of the plant ''Solanum lycopersicum'', commonly known as the tomato plant. The species originated in western South America, Mexico, and Central America. The Mexican Nahuatl word gave rise to the Spanish word ...
. New Mexico, Colorado, Arizona, and Nevada grew cantaloupes. Without refrigeration, this would have not been possible. By 1917, well-established fruit and vegetable areas that were close to eastern markets felt the pressure of competition from these distant specialized centers. Refrigeration was not limited to meat, fruit and vegetables but it also encompassed dairy product and dairy farms. In the early twentieth century, large cities got their dairy supply from farms as far as . Dairy products were not as easily transported over great distances like fruits and vegetables due to greater perishability. Refrigeration made production possible in the west far from eastern markets, so much in fact that dairy farmers could pay transportation cost and still undersell their eastern competitors. Refrigeration and the refrigerated rail gave opportunity to areas with rich soil far from natural channel of transport such as a river, valley trail or harbors.
Rise of the galactic city
"Edge city" was a term coined by
Joel Garreau
Joel Garreau (born 1948) is an American journalist, scholar, and author.
In 1981, Garreau published ''The Nine Nations of North America''. In 1991, he published '' Edge City: Life on the New Frontier''. In 2005, he published ''Radical Evolutio ...
, whereas the term "galactic city" was coined by
Lewis Mumford
Lewis Mumford (October 19, 1895 – January 26, 1990) was an American historian, sociologist, philosopher of technology, and literary critic. Particularly noted for his study of cities and urban architecture, he had a broad career as a w ...
. These terms refer to a concentration of business, shopping, and entertainment outside a traditional downtown or central business district in what had previously been a residential or rural area. There were several factors contributing to the growth of these cities such as Los Angeles, Las Vegas, Houston, and Phoenix. The factors that contributed to these large cities include reliable automobiles, highway systems, refrigeration, and agricultural production increases. Large cities such as the ones mentioned above have not been uncommon in history, but what separates these cities from the rest are that these cities are not along some natural channel of transport, or at some crossroad of two or more channels such as a trail, harbor, mountain, river, or valley. These large cities have been developed in areas that only a few hundred years ago would have been uninhabitable. Without a cost efficient way of cooling air and transporting water and food from great distances, these large cities would have never developed. The rapid growth of these cities was influenced by refrigeration and an agricultural productivity increase, allowing more distant farms to effectively feed the population.
Impact on agriculture and food production
Agriculture's role in developed countries has drastically changed in the last century due to many factors, including refrigeration. Statistics from the 2007 census gives information on the large concentration of agricultural sales coming from a small portion of the existing farms in the United States today. This is a partial result of the market created for the frozen meat trade by the first successful shipment of frozen sheep carcasses coming from New Zealand in the 1880s. As the market continued to grow, regulations on food processing and quality began to be enforced. Eventually, electricity was introduced into rural homes in the United States, which allowed refrigeration technology to continue to expand on the farm, increasing output per person. Today, refrigeration's use on the farm reduces humidity levels, avoids spoiling due to bacterial growth, and assists in preservation.
Demographics
The introduction of refrigeration and evolution of additional technologies drastically changed agriculture in the United States. During the beginning of the 20th century, farming was a common occupation and lifestyle for United States citizens, as most farmers actually lived on their farm. In 1935, there were 6.8 million farms in the United States and a population of 127 million. Yet, while the United States population has continued to climb, citizens pursuing agriculture continue to decline. Based on the 2007 US Census, less than one percent of a population of 310 million people claim farming as an occupation today. However, the increasing population has led to an increasing demand for agricultural products, which is met through a greater variety of crops, fertilizers, pesticides, and improved technology. Improved technology has decreased the risk and time involved for agricultural management and allows larger farms to increase their output per person to meet society's demand.
Meat packing and trade
Prior to 1882, the
South Island
The South Island, also officially named , is the larger of the two major islands of New Zealand in surface area, the other being the smaller but more populous North Island. It is bordered to the north by Cook Strait, to the west by the Tasman ...
of New Zealand had been experimenting with sowing grass and crossbreeding sheep, which immediately gave their farmers economic potential in the exportation of meat. In 1882, the first successful shipment of sheep carcasses was sent from
Port Chalmers
Port Chalmers is a town serving as the main port of the city of Dunedin, New Zealand. Port Chalmers lies ten kilometres inside Otago Harbour, some 15 kilometres northeast of Dunedin's city centre.
History
Early Māori settlement
The origi ...
in
Dunedin
Dunedin ( ; mi, Ōtepoti) is the second-largest city in the South Island of New Zealand (after Christchurch), and the principal city of the Otago region. Its name comes from , the Scottish Gaelic name for Edinburgh, the capital of Scotland. Th ...
, New Zealand, to
London
London is the capital and largest city of England and the United Kingdom, with a population of just under 9 million. It stands on the River Thames in south-east England at the head of a estuary down to the North Sea, and has been a majo ...
. By the 1890s, the frozen meat trade became increasingly more profitable in New Zealand, especially in
Canterbury
Canterbury (, ) is a City status in the United Kingdom, cathedral city and UNESCO World Heritage Site, situated in the heart of the City of Canterbury local government district of Kent, England. It lies on the River Stour, Kent, River Stour.
...
, where 50% of exported sheep carcasses came from in 1900. It wasn't long before Canterbury meat was known for the highest quality, creating a demand for New Zealand meat around the world. In order to meet this new demand, the farmers improved their feed so sheep could be ready for the slaughter in only seven months. This new method of shipping led to an economic boom in New Zealand by the mid 1890s.
In the United States, the Meat Inspection Act of 1891 was put in place in the United States because local butchers felt the refrigerated railcar system was unwholesome. When meat packing began to take off, consumers became nervous about the quality of the meat for consumption.
Upton Sinclair
Upton Beall Sinclair Jr. (September 20, 1878 – November 25, 1968) was an American writer, muckraker, political activist and the 1934 Democratic Party nominee for governor of California who wrote nearly 100 books and other works in seve ...
's 1906 novel ''
The Jungle
''The Jungle'' is a 1906 novel by the American journalist and novelist Upton Sinclair. Sinclair's primary purpose in describing the meat industry and its working conditions was to advance socialism in the United States. However, most readers we ...
'' brought negative attention to the meat packing industry, by drawing to light unsanitary working conditions and processing of diseased animals. The book caught the attention of President
Theodore Roosevelt
Theodore Roosevelt Jr. ( ; October 27, 1858 – January 6, 1919), often referred to as Teddy or by his initials, T. R., was an American politician, statesman, soldier, conservationist, naturalist, historian, and writer who served as the 26t ...
, and
the 1906 Meat Inspection Act was put into place as an amendment to the Meat Inspection Act of 1891. This new act focused on the quality of the meat and environment it is processed in.
Electricity in rural areas
In the early 1930s, 90 percent of the urban population of the United States
had electric power, in comparison to only 10 percent of rural homes. At the time, power companies did not feel that extending power to rural areas (
rural electrification
Rural electrification is the process of bringing electrical power to rural and remote areas. Rural communities are suffering from colossal market failures as the national grids fall short of their demand for electricity. As of 2017, over 1 billion ...
) would produce enough profit to make it worth their while. However, in the midst of the
Great Depression
The Great Depression (19291939) was an economic shock that impacted most countries across the world. It was a period of economic depression that became evident after a major fall in stock prices in the United States. The economic contagio ...
, President
Franklin D. Roosevelt
Franklin Delano Roosevelt (; ; January 30, 1882April 12, 1945), often referred to by his initials FDR, was an American politician and attorney who served as the 32nd president of the United States from 1933 until his death in 1945. As the ...
realized that rural areas would continue to lag behind urban areas in both poverty and production if they were not electrically wired. On May 11, 1935, the president signed an executive order called the
Rural Electrification Administration
The United States Rural Utilities Service (RUS) administers programs that provide infrastructure or infrastructure improvements to rural communities. These include water and waste treatment, electric power, and telecommunications services. it is ...
, also known as REA. The agency provided loans to fund electric infrastructure in the rural areas. In just a few years, 300,000 people in rural areas of the United States had received power in their homes.
While electricity dramatically improved working conditions on farms, it also had a large impact on the safety of food production. Refrigeration systems were introduced to the farming and
food distribution processes, which helped in
food preservation
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
and
kept food supplies safe. Refrigeration also allowed for shipment of perishable commodities throughout the United States. As a result, United States farmers quickly became the most productive in the world, and entire new
food systems
The term food system describes the interconnected systems and processes that influence nutrition, food, health, community development, and agriculture. A food system includes all processes and infrastructure involved in feeding a population: growi ...
arose.
Farm use
In order to reduce humidity levels and spoiling due to bacterial growth, refrigeration is used for meat, produce, and dairy processing in farming today. Refrigeration systems are used the heaviest in the warmer months for farming produce, which must be cooled as soon as possible in order to meet quality standards and increase the shelf life. Meanwhile, dairy farms refrigerate milk year round to avoid spoiling.
Effects on lifestyle and diet
In the late 19th Century and into the very early 20th Century, except for staple foods (sugar, rice, and beans) that needed no refrigeration, the available foods were affected heavily by the seasons and what could be grown locally. Refrigeration has removed these limitations. Refrigeration played a large part in the feasibility and then popularity of the modern supermarket. Fruits and vegetables out of season, or grown in distant locations, are now available at relatively low prices. Refrigerators have led to a huge increase in meat and dairy products as a portion of overall supermarket sales. As well as changing the goods purchased at the market, the ability to store these foods for extended periods of time has led to an increase in leisure time. Prior to the advent of the household refrigerator, people would have to shop on a daily basis for the supplies needed for their meals.
Impact on nutrition
The introduction of refrigeration allowed for the hygienic handling and storage of perishables, and as such, promoted output growth, consumption, and the availability of nutrition. The change in our method of food preservation moved us away from salts to a more manageable sodium level. The ability to move and store perishables such as meat and dairy led to a 1.7% increase in dairy consumption and overall protein intake by 1.25% annually in the US after the 1890s.
People were not only consuming these perishables because it became easier for they themselves to store them, but because the innovations in refrigerated transportation and storage led to less spoilage and waste, thereby driving the prices of these products down. Refrigeration accounts for at least 5.1% of the increase in adult stature (in the US) through improved nutrition, and when the indirect effects associated with improvements in the quality of nutrients and the reduction in illness is additionally factored in, the overall impact becomes considerably larger.
Recent studies have also shown a negative relationship between the number of refrigerators in a household and the rate of gastric cancer mortality.
Current applications of refrigeration
Probably the most widely used current applications of refrigeration are for
air conditioning
Air conditioning, often abbreviated as A/C or AC, is the process of removing heat from an enclosed space to achieve a more comfortable interior environment (sometimes referred to as 'comfort cooling') and in some cases also strictly controlling ...
of private homes and public buildings, and refrigerating foodstuffs in homes, restaurants and large storage warehouses. The use of
refrigerators and walk-in coolers and freezers in kitchens, factories and warehouses for storing and processing fruits and vegetables has allowed adding fresh salads to the modern diet year round, and storing fish and meats safely for long periods.
The optimum temperature range for perishable food storage is .
[Keep your fridge-freezer clean and ice-free](_blank)
''BBC''. 30 April 2008
In commerce and manufacturing, there are many uses for refrigeration. Refrigeration is used to liquefy gases –
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
,
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
,
propane, and
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
, for example. In compressed air purification, it is used to
condense
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
water vapor from compressed air to reduce its moisture content. In
oil refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, lique ...
,
chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transform ...
s, and
petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sou ...
plants, refrigeration is used to maintain certain processes at their needed low temperatures (for example, in
alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
of
butene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
s and
butane to produce a high-
octane
Octane is a hydrocarbon and an alkane with the chemical formula , and the condensed structural formula . Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-Tri ...
gasoline component). Metal workers use refrigeration to temper steel and cutlery. When transporting temperature-sensitive foodstuffs and other materials by trucks, trains, airplanes and seagoing vessels, refrigeration is a necessity.
Dairy products are constantly in need of refrigeration, and it was only discovered in the past few decades that eggs needed to be refrigerated during shipment rather than waiting to be refrigerated after arrival at the grocery store. Meats, poultry and fish all must be kept in climate-controlled environments before being sold. Refrigeration also helps keep fruits and vegetables edible longer.
One of the most influential uses of refrigeration was in the development of the
sushi
is a Japanese dish of prepared , usually with some sugar and salt, accompanied by a variety of , such as seafood, often raw, and vegetables. Styles of sushi and its presentation vary widely, but the one key ingredient is "sushi rice," also ...
/
sashimi
is a Japanese delicacy consisting of fresh raw fish or meat sliced into thin pieces and often eaten with soy sauce.
Origin
The word ''sashimi'' means "pierced body", i.e. " 刺身" = ''sashimi'', where 刺 し = ''sashi'' (pierced, stu ...
industry in Japan. Before the discovery of refrigeration, many sushi connoisseurs were at risk of contracting diseases. The dangers of unrefrigerated sashimi were not brought to light for decades due to the lack of research and healthcare distribution across rural Japan. Around mid-century, the
Zojirushi
The is a Japanese multinational manufacturer and marketer of vacuum flasks, beverage dispensers, and consumer electronics including bread machines, electric kettles, hot water dispensers, electric water boilers and rice cookers. It has a ...
corporation, based in Kyoto, made breakthroughs in refrigerator designs, making refrigerators cheaper and more accessible for restaurant proprietors and the general public.
Methods of refrigeration
Methods of refrigeration can be classified as ''non-cyclic'', ''cyclic'', ''thermoelectric'' and ''magnetic''.
Non-cyclic refrigeration
This refrigeration method cools a contained area by melting ice, or by sublimating
dry ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily ...
. Perhaps the simplest example of this is a portable cooler, where items are put in it, then ice is poured over the top. Regular ice can maintain temperatures near, but not below the freezing point, unless salt is used to cool the ice down further (as in a
traditional ice-cream maker). Dry ice can reliably bring the temperature well below water freezing point.
Cyclic refrigeration
This consists of a refrigeration cycle, where heat is removed from a low-temperature space or source and rejected to a high-temperature sink with the help of external work, and its inverse, the
thermodynamic power cycle. In the power cycle, heat is supplied from a high-temperature source to the engine, part of the heat being used to produce work and the rest being rejected to a low-temperature sink. This satisfies the
second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects ( ...
.
A ''refrigeration cycle'' describes the changes that take place in the refrigerant as it alternately absorbs and rejects heat as it circulates through a
refrigerator. It is also applied to heating, ventilation, and air conditioning
HVACR
Heating, ventilation, and air conditioning (HVAC) is the use of various technologies to control the temperature, humidity, and purity of the air in an enclosed space. Its goal is to provide thermal comfort and acceptable indoor air quality. HV ...
work, when describing the "process" of refrigerant flow through an HVACR unit, whether it is a packaged or split system.
Heat naturally flows from hot to cold.
Work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an animal t ...
is applied to cool a living space or storage volume by pumping heat from a lower temperature heat source into a higher temperature heat sink.
Insulation
Insulation may refer to:
Thermal
* Thermal insulation, use of materials to reduce rates of heat transfer
** List of insulation materials
** Building insulation, thermal insulation added to buildings for comfort and energy efficiency
*** Insulated ...
is used to reduce the work and
energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
needed to achieve and maintain a lower temperature in the cooled space. The operating principle of the refrigeration cycle was described mathematically by
Sadi Carnot in 1824 as a
heat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state ...
.
The most common types of refrigeration systems use the reverse-Rankine
vapor-compression refrigeration
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings ...
cycle, although
absorption heat pump
An absorption heat pump (AHP) is a heat pump driven by thermal energy such as combustion of natural gas, steam solar-heated water, air or geothermal-heated water differently from compression heat pumps that are driven by mechanical energy.
AHPs ...
s are used in a minority of applications.
Cyclic refrigeration can be classified as:
#Vapor cycle, and
#Gas cycle
Vapor cycle refrigeration can further be classified as:
#
Vapor-compression refrigeration
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings ...
#Sorption Refrigeration
##
Vapor-absorption refrigeration
##
Adsorption refrigeration
Vapor-compression cycle


The vapor-compression cycle is used in most household refrigerators as well as in many large commercial and
industrial refrigeration systems. Figure 1 provides a schematic diagram of the components of a typical vapor-compression refrigeration system.
The
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
of the cycle can be analyzed on a diagram as shown in Figure 2. In this cycle, a circulating refrigerant such as a low boiling hydrocarbon or
hydrofluorocarbons
Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditi ...
enters the
compressor as a vapour. From point 1 to point 2, the vapor is compressed at constant
entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
and exits the compressor as a vapor at a higher temperature, but still below the
vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phas ...
at that temperature. From point 2 to point 3 and on to point 4, the vapor travels through the
condenser which cools the vapour until it starts condensing, and then condenses the vapor into a liquid by removing additional heat at constant pressure and temperature. Between points 4 and 5, the liquid refrigerant goes through the
expansion valve (also called a throttle valve) where its pressure abruptly decreases, causing
flash evaporation
Flash evaporation (or partial evaporation) is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest un ...
and auto-refrigeration of, typically, less than half of the liquid.
That results in a mixture of liquid and vapour at a lower temperature and pressure as shown at point 5. The cold liquid-vapor mixture then travels through the evaporator coil or tubes and is completely vaporized by cooling the warm air (from the space being refrigerated) being blown by a fan across the evaporator coil or tubes. The resulting refrigerant vapour returns to the compressor inlet at point 1 to complete the thermodynamic cycle.
The above discussion is based on the ideal vapour-compression refrigeration cycle, and does not take into account real-world effects like frictional pressure drop in the system, slight
thermodynamic irreversibility during the compression of the refrigerant vapor, or
non-ideal gas behavior, if any. Vapor compression refrigerators can be arranged in two stages in
cascade refrigeration
A cascade refrigeration cycle is a multi-stage thermodynamic cycle. An example two-stage process is shown at right. (Bottom on mobile) The cascade cycle is often employed for devices such as ULT freezer
An ultra low temperature (ULT) freezer is ...
systems, with the second stage cooling the condenser of the first stage. This can be used for achieving very low temperatures.
More information about the design and performance of vapor-compression refrigeration systems is available in the classic ''
Perry's Chemical Engineers' Handbook
''Perry's Chemical Engineers' Handbook'' (also known as ''Perry's Handbook'', ''Perry's'', or ''The Chemical Engineer's Bible'') was first published in 1934 and the most current ninth edition was published in July 2018. It has been a source of c ...
''.
Sorption cycle
=Absorption cycle
=
In the early years of the twentieth century, the vapor absorption cycle using water-ammonia systems or
LiBr-water was popular and widely used. After the development of the vapor compression cycle, the vapor absorption cycle lost much of its importance because of its low
coefficient of performance
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy ( ...
(about one fifth of that of the vapor compression cycle). Today, the vapor absorption cycle is used mainly where fuel for heating is available but electricity is not, such as in
recreational vehicles
A recreational vehicle, often abbreviated as RV, is a motor vehicle or trailer that includes living quarters designed for accommodation. Types of RVs include motorhomes, campervans, coaches, caravans (also known as travel trailers and camper ...
that carry
LP gas
Liquefied petroleum gas (LPG or LP gas) is a fuel gas which contains a flammable mixture of hydrocarbon gases, specifically propane, propylene, butylene, isobutane and n-butane.
LPG is used as a fuel gas in heating appliances, cooking ...
. It is also used in industrial environments where plentiful waste heat overcomes its inefficiency.
The absorption cycle is similar to the compression cycle, except for the method of raising the pressure of the refrigerant vapor. In the absorption system, the compressor is replaced by an absorber which dissolves the refrigerant in a suitable liquid, a liquid pump which raises the pressure and a generator which, on heat addition, drives off the refrigerant vapor from the high-pressure liquid. Some work is needed by the liquid pump but, for a given quantity of refrigerant, it is much smaller than needed by the compressor in the vapor compression cycle. In an absorption refrigerator, a suitable combination of refrigerant and absorbent is used. The most common combinations are ammonia (refrigerant) with water (absorbent), and water (refrigerant) with lithium bromide (absorbent).
=Adsorption cycle
=
The main difference with absorption cycle, is that in adsorption cycle, the refrigerant (adsorbate) could be ammonia, water,
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
, etc., while the adsorbent is a solid, such as
silica gel
Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other l ...
,
activated carbon, or
zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These p ...
, unlike in the absorption cycle where absorbent is liquid.
The reason adsorption refrigeration technology has been extensively researched in recent 30 years lies in that the operation of an adsorption refrigeration system is often noiseless, non-corrosive and environment friendly.
Gas cycle
When the
working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, ...
is a gas that is compressed and expanded but doesn't change phase, the refrigeration cycle is called a ''gas cycle''.
Air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
is most often this working fluid. As there is no condensation and evaporation intended in a gas cycle, components corresponding to the condenser and evaporator in a vapor compression cycle are the hot and cold gas-to-gas
heat exchanger
A heat exchanger is a system used to transfer heat between a source and a working fluid. Heat exchangers are used in both cooling and heating processes. The fluids may be separated by a solid wall to prevent mixing or they may be in direct conta ...
s in gas cycles.
The gas cycle is less efficient than the vapor compression cycle because the gas cycle works on the reverse
Brayton cycle
The Brayton cycle is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. The original Brayton engines used a piston compressor and piston expander, but modern gas tu ...
instead of the reverse
Rankine cycle
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat sourc ...
. As such, the working fluid does not receive and reject heat at constant temperature. In the gas cycle, the refrigeration effect is equal to the product of the specific heat of the gas and the rise in temperature of the gas in the low temperature side. Therefore, for the same cooling load, a gas refrigeration cycle needs a large mass flow rate and is bulky.
Because of their lower efficiency and larger bulk, ''air cycle'' coolers are not often used nowadays in terrestrial cooling devices. However, the
air cycle machine
An air cycle machine (ACM) is the refrigeration unit of the environmental control system (ECS) used in pressurized gas turbine-powered aircraft. Normally an aircraft has two or three of these ACM. Each ACM and its components are often referred as ...
is very common on
gas turbine
A gas turbine, also called a combustion turbine, is a type of continuous flow internal combustion engine. The main parts common to all gas turbine engines form the power-producing part (known as the gas generator or core) and are, in the directio ...
-powered jet
aircraft
An aircraft is a vehicle that is able to fly by gaining support from the air. It counters the force of gravity by using either static lift or by using the dynamic lift of an airfoil, or in a few cases the downward thrust from jet engines ...
as cooling and ventilation units, because compressed air is readily available from the engines' compressor sections. Such units also serve the purpose of pressurizing the aircraft.
Thermoelectric refrigeration
Thermoelectric cooling
Thermoelectric cooling uses the Peltier effect to create a heat flux at the junction of two different types of materials. A Peltier cooler, heater, or thermoelectric heat pump is a solid-state active heat pump which transfers heat from one side o ...
uses the
Peltier effect
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when ...
to create a heat
flux between the junction of two types of material. This effect is commonly used in camping and portable coolers and for cooling electronic components and small instruments. Peltier coolers are often used where a traditional vapor-compression cycle refrigerator would be impractical or take up too much space, and in cooled image sensors as an easy, compact and lightweight, if inefficient, way to achieve very low temperatures, using two or more stage peltier coolers arranged in a
cascade refrigeration
A cascade refrigeration cycle is a multi-stage thermodynamic cycle. An example two-stage process is shown at right. (Bottom on mobile) The cascade cycle is often employed for devices such as ULT freezer
An ultra low temperature (ULT) freezer is ...
configuration, meaning that two or more Peltier elements are stacked on top of each other, with each stage being larger than the one before it, in order to extract more heat and waste heat generated by the previous stages. Peltier cooling has a low COP (efficiency) when compared with that of the vapor-compression cycle, so it emits more waste heat (heat generated by the Peltier element or cooling mechanism) and consumes more power for a given cooling capacity.
Magnetic refrigeration
Magnetic refrigeration, or
adiabatic demagnetization
In classical electromagnetism, magnetization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material. Movement within this field is described by direction and is either Axial or Di ...
, is a cooling technology based on the magnetocaloric effect, an
intrinsic property
In science and engineering, an intrinsic property is a property of a specified subject that exists itself or within the subject. An extrinsic property is not essential or inherent to the subject that is being characterized. For example, mass ...
of magnetic solids. The refrigerant is often a
paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
, such as
cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
. The active
magnetic
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particle ...
dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system ...
s in this case are those of the
electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or ...
s of the paramagnetic atoms.
A strong magnetic field is applied to the refrigerant, forcing its various magnetic dipoles to align and putting these degrees of freedom of the refrigerant into a state of lowered
entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
. A heat sink then absorbs the heat released by the refrigerant due to its loss of entropy. Thermal contact with the heat sink is then broken so that the system is insulated, and the magnetic field is switched off. This increases the heat capacity of the refrigerant, thus decreasing its temperature below the temperature of the heat sink.
Because few materials exhibit the needed properties at room temperature, applications have so far been limited to
cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
and research.
Other methods
Other methods of refrigeration include the
air cycle machine
An air cycle machine (ACM) is the refrigeration unit of the environmental control system (ECS) used in pressurized gas turbine-powered aircraft. Normally an aircraft has two or three of these ACM. Each ACM and its components are often referred as ...
used in aircraft; the
vortex tube
The vortex tube, also known as the Ranque-Hilsch vortex tube, is a mechanical device that separates a compressed gas into hot and cold streams. The gas emerging from the hot end can reach temperatures of , and the gas emerging from the cold end ...
used for spot cooling, when compressed air is available; and
thermoacoustic refrigeration
Thermoacoustic engines (sometimes called "TA engines") are thermoacoustic devices which use high-amplitude sound waves to pump heat from one place to another (this requires work, which is provided by the loudspeaker) or use a heat difference to ...
using sound waves in a pressurized gas to drive heat transfer and heat exchange;
steam jet cooling popular in the early 1930s for air conditioning large buildings; thermoelastic cooling using a smart metal alloy stretching and relaxing. Many
Stirling cycle heat engines can be run backwards to act as a refrigerator, and therefore these engines have a niche use in
cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
. In addition, there are other types of
cryocoolers
A refrigerator designed to reach cryogenic temperatures (below ) is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as lo ...
such as Gifford-McMahon coolers, Joule-Thomson coolers,
pulse-tube refrigerators and, for temperatures between 2 mK and 500 mK,
dilution refrigerator
A 3He/4He dilution refrigerator is a cryogenic device that provides continuous cooling to temperatures as low as 2 mK, with no moving parts in the low-temperature region. The cooling power is provided by the heat of mixing of the Hel ...
s.
Elastocaloric refrigeration
Another potential solid-state refrigeration technique and a relatively new area of study comes from a special property of
super elastic materials. These materials undergo a temperature change when experiencing an applied mechanical
stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
(called the elastocaloric effect). Since super elastic materials deform reversibly at high
strains, the material experiences a flattened
elastic
Elastic is a word often used to describe or identify certain types of elastomer, elastic used in garments or stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rubber used to hold objects togeth ...
region in its
stress-strain curve caused by a resulting phase transformation from an
austenitic
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 ...
to a
martensitic
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.
Properties
Marte ...
crystal phase.
When a super elastic material experiences a stress in the austenitic phase, it undergoes an
exothermic phase transformation
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
to the martensitic phase, which causes the material to heat up. Removing the stress reverses the process, restores the material to its austenitic phase, and
absorbs heat from the surroundings cooling down the material.
The most appealing part of this research is how potentially energy efficient and environmentally friendly this cooling technology is. The different materials used, commonly
shape-memory alloy
In metallurgy, a shape-memory alloy (SMA) is an alloy that can be deformed when cold but returns to its pre-deformed ("remembered") shape when heated. It may also be called memory metal, memory alloy, smart metal, smart alloy, or muscle wire.
P ...
s, provide a non-toxic source of emission free refrigeration. The most commonly studied materials studied are shape-memory alloys, like
nitinol
Nickel titanium, also known as Nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages. Different alloys are named according to the weight percentage of nickel; e.g., Nitinol 55 and ...
and Cu-Zn-Al. Nitinol is of the more promising alloys with output heat at about 66 J/cm
3 and a temperature change of about 16–20 K. Due to the difficulty in manufacturing some of the shape memory alloys, alternative materials like
natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
have been studied. Even though rubber may not give off as much heat per volume (12 J/cm
3 ) as the shape memory alloys, it still generates a comparable temperature change of about 12 K and operates at a suitable temperature range, low stresses, and low cost.
The main challenge however comes from potential energy losses in the form of
hysteresis, often associated with this process. Since most of these losses comes from incompatibilities between the two phases, proper alloy tuning is necessary to reduce losses and increase reversibility and
efficiency. Balancing the transformation strain of the material with the energy losses enables a large elastocaloric effect to occur and potentially a new alternative for refrigeration.
Fridge Gate
The Fridge Gate method is a theoretical application of using a single logic gate to drive a refrigerator in the most energy efficient way possible without violating the laws of thermodynamics. It operates on the fact that there are two energy states in which a particle can exist: the ground state and the excited state. The excited state carries a little more energy than the ground state, small enough so that the transition occurs with high probability. There are three components or particle types associated with the fridge gate. The first is on the interior of the fridge, the second on the outside and the third is connected to a power supply which heats up every so often that it can reach the E state and replenish the source. In the cooling step on the inside of the fridge, the g state particle absorbs energy from ambient particles, cooling them, and itself jumping to the e state. In the second step, on the outside of the fridge where the particles are also at an e state, the particle falls to the g state, releasing energy and heating the outside particles. In the third and final step, the power supply moves a particle at the e state, and when it falls to the g state it induces an energy-neutral swap where the interior e particle is replaced by a new g particle, restarting the cycle.
Passive systems
When combining a
passive daytime radiative cooling
Passive daytime radiative cooling (PDRC) is a renewable cooling method proposed as a solution to global warming of enhancing terrestrial heat flow to outer space through the installation of thermally-emissive surfaces on Earth that require zer ...
system with
thermal insulation
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with s ...
and
evaporative cooling
An evaporative cooler (also known as evaporative air conditioner, swamp cooler, swamp box, desert cooler and wet air cooler) is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning sy ...
, one study found a 300% increase in ambient cooling power when compared to a stand-alone radiative cooling surface, which could extend the
shelf life
Shelf life is the length of time that a commodity may be stored without becoming unfit for use, consumption, or sale. In other words, it might refer to whether a commodity should no longer be on a pantry shelf (unfit for use), or no longer on a ...
of food by 40% in
humid climates and 200% in
desert climates without refrigeration. The system's evaporative cooling layer would require water "re-charges" every 10 days to a month in humid areas and every 4 days in hot and dry areas.
Capacity ratings
The refrigeration capacity of a refrigeration system is the product of the
evaporator
An evaporator is a device used to turn the liquid form of a chemical substance, such as water, into a vapor.
Uses
Air conditioning and refrigeration
Some air conditioners and refrigerators use a compressed liquid with a low boiling point, su ...
s’
enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
rise and the evaporators’
mass flow rate
In physics and engineering, mass flow rate is the mass of a substance which passes per unit of time. Its unit is kilogram per second in SI units, and slug per second or pound per second in US customary units. The common symbol is \dot ('' ...
. The measured capacity of refrigeration is often dimensioned in the unit of kW or BTU/h. Domestic and commercial refrigerators may be rated in kJ/s, or Btu/h of cooling. For commercial and industrial refrigeration systems, the kilowatt (kW) is the basic unit of refrigeration, except in North America, where both
ton of refrigeration
A ton of refrigeration (TR or TOR), also called a refrigeration ton (RT), is a unit of power used in some countries (especially in North America) to describe the heat-extraction capacity of refrigeration and air conditioning equipment.
It was o ...
and BTU/h are used.
A refrigeration system's
coefficient of performance
The coefficient of performance or COP (sometimes CP or CoP) of a heat pump, refrigerator or air conditioning system is a ratio of useful heating or cooling provided to work (energy) required. Higher COPs equate to higher efficiency, lower energy ( ...
(CoP) is very important in determining a system's overall efficiency. It is defined as refrigeration capacity in kW divided by the energy input in kW. While CoP is a very simple measure of performance, it is typically not used for industrial refrigeration in North America. Owners and manufacturers of these systems typically use performance factor (PF). A system's PF is defined as a system's energy input in horsepower divided by its refrigeration capacity in TR. Both CoP and PF can be applied to either the entire system or to system components. For example, an individual compressor can be rated by comparing the energy needed to run the compressor versus the expected refrigeration capacity based on inlet volume flow rate. It is important to note that both CoP and PF for a refrigeration system are only defined at specific operating conditions, including temperatures and thermal loads. Moving away from the specified operating conditions can dramatically change a system's performance.
Air conditioning systems used in residential application typically use
SEER
In the United States, the efficiency of air conditioners is often rated by the seasonal energy efficiency ratio (SEER) which is defined by the Air Conditioning, Heating, and Refrigeration Institute, a trade association, in its 2008 standard AHR ...
(Seasonal Energy Efficiency Ratio)for the energy performance rating. Air conditioning systems for commercial application often use EER (
Energy Efficiency Ratio
In the United States, the efficiency of air conditioners is often rated by the seasonal energy efficiency ratio (SEER) which is defined by the Air Conditioning, Heating, and Refrigeration Institute, a trade association, in its 2008 standard AHR ...
) and IEER (Integrated Energy Efficiency Ratio) for the energy efficiency performance rating.
See also
*
Air conditioning
Air conditioning, often abbreviated as A/C or AC, is the process of removing heat from an enclosed space to achieve a more comfortable interior environment (sometimes referred to as 'comfort cooling') and in some cases also strictly controlling ...
*
Auto-defrost
Auto-defrost, automatic defrost or self-defrosting is a technique which regularly defrosts the evaporator in a refrigerator or freezer. Appliances using this technique are often called frost free, frostless, or no-frost.
Mechanism
The defrost ...
*
Beef ring
*
Carnot heat engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1 ...
*
Cold chain
A cold chain is a low temperature-controlled supply chain network. An unbroken cold chain is an uninterrupted series of refrigerated production, storage and distribution activities, along with associated equipment and logistics, which maintain qu ...
*
Coolgardie safe
The Coolgardie safe is a low-tech food storage unit, using evaporative cooling to prolong the life of whatever edibles are kept in it. It applies the basic principle of heat transfer which occurs during evaporation of water (see latent heat and ...
*
Cryocooler
A refrigerator designed to reach cryogenic temperatures (below ) is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as l ...
*
Darcy friction factor formulae
In fluid dynamics, the Darcy friction factor formulae are equations that allow the calculation of the Darcy friction factor, a dimensionless quantity used in the Darcy–Weisbach equation, for the description of friction losses in pipe flow as w ...
*
Einstein refrigerator
The Einstein–Szilard or Einstein refrigerator is an absorption refrigerator which has no moving parts, operates at constant pressure, and requires only a heat source to operate. It was jointly invented in 1926 by Albert Einstein and his form ...
*
Freezer
A refrigerator, colloquially fridge, is a commercial and home appliance consisting of a thermally insulated compartment and a heat pump (mechanical, electronic or chemical) that transfers heat from its inside to its external environment so th ...
*
Heat pump
A heat pump is a device that can heat a building (or part of a building) by transferring thermal energy from the outside using a refrigeration cycle. Many heat pumps can also operate in the opposite direction, cooling the building by removing ...
*
Heat pump and refrigeration cycle
Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that allows for the transmission of heat from one locat ...
*
Heating, ventilation, and air conditioning
Heating, ventilation, and air conditioning (HVAC) is the use of various technologies to control the temperature, humidity, and purity of the air in an enclosed space. Its goal is to provide thermal comfort and acceptable indoor air quality. HV ...
(HVAC, HVACR)
*
Icebox
An icebox (also called a cold closet) is a compact non-mechanical refrigerator which was a common early-twentieth-century kitchen appliance before the development of safely powered refrigeration devices. Before the development of electric refrige ...
*
Icyball
Icyball is a name given to two early refrigerators, one made by Australian Sir Edward Hallstrom in 1923, and the other design patented by David Forbes Keith of Toronto (filed 1927, granted 1929), and manufactured by American Powel Crosley Jr., ...
*
Joule–Thomson effect
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a ''real'' gas or liquid (as differentiated from an ideal gas) when it is forced through a valv ...
*
Laser cooling
Laser cooling includes a number of techniques in which atoms, molecules, and small mechanical systems are cooled, often approaching temperatures near absolute zero. Laser cooling techniques rely on the fact that when an object (usually an atom) ...
*
Pot-in-pot refrigerator
A pot-in-pot refrigerator, clay pot cooler or zeer ( ar, زير) is an evaporative cooling refrigeration device which does not use electricity. It uses a porous outer clay pot (lined with wet sand) containing an inner pot (which can be glazed t ...
*
Pumpable ice technology
Pumpable ice technology (PIT) uses thin liquids, with the cooling capacity of ice. Pumpable ice is typically a slurry of ice crystals or particles ranging from 5 micrometers to 1 cm in diameter and transported in brine, seawater, food liqui ...
*
Quantum refrigerators A quantum heat engine is a device that generates power from the heat flow between hot and cold reservoirs.
The operation mechanism of the engine can be described by the laws of quantum mechanics.
The first realization of a quantum heat engine was ...
*
Redundant refrigeration system
*
Reefer ship
A reefer ship is a refrigerated cargo ship typically used to transport perishable cargo, which require temperature-controlled handling, such as fruits, meat, vegetables, dairy products, and similar items.
Description
''Types of reefers:'' Re ...
*
Refrigerant
*
Refrigerated container
A refrigerated container or reefer is an intermodal container (shipping container) used in intermodal freight transport that is capable of refrigeration for the transportation of temperature-sensitive, perishable cargo such as fruits, vegetabl ...
*
Refrigerator
*
Refrigerator car
A refrigerator car (or "reefer") is a refrigerated boxcar (U.S.), a piece of railroad rolling stock designed to carry perishable freight at specific temperatures. Refrigerator cars differ from simple insulated boxcars and ventilated boxcars (co ...
*
Refrigerator truck
A refrigerator truck or chiller lorry (also called a Reefer), is a van or truck designed to carry perishable freight at low temperatures. Most long-distance refrigerated transport by truck is done in articulated trucks pulling refrigerated semi- ...
*
Seasonal energy efficiency ratio
In the United States, the efficiency of air conditioners is often rated by the seasonal energy efficiency ratio (SEER) which is defined by the Air Conditioning, Heating, and Refrigeration Institute, a trade association, in its 2008 standard AHRI ...
(SEER)
*
Steam jet cooling
*
Thermoacoustics
Thermoacoustics is the interaction between temperature, density and pressure variations of acoustic waves. Thermoacoustic heat engines can readily be driven using solar energy or waste heat and they can be controlled using proportional control. T ...
*
Vapor-compression refrigeration
Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings ...
*
Working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, ...
*
World Refrigeration Day
References
Further reading
*''Refrigeration volume'',
ASHRAE Handbook
The ASHRAE Handbook is the four-volume flagship publication of the nonprofit technical organization ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers). This Handbook is considered the most comprehensive and autho ...
, ASHRAE, Inc., Atlanta, GA
*Stoecker and Jones, ''Refrigeration and Air Conditioning'', Tata-McGraw Hill Publishers
*Mathur, M.L., Mehta, F.S., ''Thermal Engineering'' Vol II
*MSN Encarta Encyclopedia
*
*
*
*
External links
Green Cooling Initiative on alternative natural refrigerants cooling technologies"The Refrigeration", from frigokeyAmerican Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE)International Institute of Refrigeration (IIR)British Institute of Refrigeration
* ttps://www.ior.org.uk/ Institute of Refrigeration
{{Authority control
Chemical processes
Cooling technology
Food preservation
Heating, ventilation, and air conditioning
Thermodynamics
 The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology Refrigeration can be considered an artificial, or human-made,
The term refrigeration refers to the process of removing heat from an enclosed space or substance for the purpose of lowering the temperature.International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.phpASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology Refrigeration can be considered an artificial, or human-made,  Before 1830, few Americans used ice to refrigerate foods due to a lack of ice-storehouses and iceboxes. As these two things became more widely available, individuals used axes and saws to harvest ice for their storehouses. This method proved to be difficult, dangerous, and certainly did not resemble anything that could be duplicated on a commercial scale.
Despite the difficulties of harvesting ice, Frederic Tudor thought that he could capitalize on this new commodity by harvesting ice in New England and shipping it to the Caribbean islands as well as the southern states. In the beginning, Tudor lost thousands of dollars, but eventually turned a profit as he constructed icehouses in Charleston, Virginia and in the Cuban port town of Havana. These icehouses as well as better insulated ships helped reduce ice wastage from 66% to 8%. This efficiency gain influenced Tudor to expand his ice market to other towns with icehouses such as New Orleans and Savannah. This ice market further expanded as harvesting ice became faster and cheaper after one of Tudor's suppliers, Nathaniel Wyeth, invented a horse-drawn ice cutter in 1825. This invention as well as Tudor's success inspired others to get involved in the
Before 1830, few Americans used ice to refrigerate foods due to a lack of ice-storehouses and iceboxes. As these two things became more widely available, individuals used axes and saws to harvest ice for their storehouses. This method proved to be difficult, dangerous, and certainly did not resemble anything that could be duplicated on a commercial scale.
Despite the difficulties of harvesting ice, Frederic Tudor thought that he could capitalize on this new commodity by harvesting ice in New England and shipping it to the Caribbean islands as well as the southern states. In the beginning, Tudor lost thousands of dollars, but eventually turned a profit as he constructed icehouses in Charleston, Virginia and in the Cuban port town of Havana. These icehouses as well as better insulated ships helped reduce ice wastage from 66% to 8%. This efficiency gain influenced Tudor to expand his ice market to other towns with icehouses such as New Orleans and Savannah. This ice market further expanded as harvesting ice became faster and cheaper after one of Tudor's suppliers, Nathaniel Wyeth, invented a horse-drawn ice cutter in 1825. This invention as well as Tudor's success inspired others to get involved in the  The history of artificial refrigeration began when Scottish professor
The history of artificial refrigeration began when Scottish professor  The first
The first 
 In 1842,
In 1842, 
 During the early 1800s, consumers preserved their food by storing food and ice purchased from ice harvesters in iceboxes. In 1803, Thomas Moore patented a metal-lined butter-storage tub which became the prototype for most iceboxes. These iceboxes were used until nearly 1910 and the technology did not progress. In fact, consumers that used the icebox in 1910 faced the same challenge of a moldy and stinky icebox that consumers had in the early 1800s.
General Electric (GE) was one of the first companies to overcome these challenges. In 1911, GE released a household refrigeration unit that was powered by gas. The use of gas eliminated the need for an electric compressor motor and decreased the size of the refrigerator. However, electric companies that were customers of GE did not benefit from a gas-powered unit. Thus, GE invested in developing an electric model. In 1927, GE released the Monitor Top, the first refrigerator to run on electricity.
In 1930, Frigidaire, one of GE's main competitors, synthesized
During the early 1800s, consumers preserved their food by storing food and ice purchased from ice harvesters in iceboxes. In 1803, Thomas Moore patented a metal-lined butter-storage tub which became the prototype for most iceboxes. These iceboxes were used until nearly 1910 and the technology did not progress. In fact, consumers that used the icebox in 1910 faced the same challenge of a moldy and stinky icebox that consumers had in the early 1800s.
General Electric (GE) was one of the first companies to overcome these challenges. In 1911, GE released a household refrigeration unit that was powered by gas. The use of gas eliminated the need for an electric compressor motor and decreased the size of the refrigerator. However, electric companies that were customers of GE did not benefit from a gas-powered unit. Thus, GE invested in developing an electric model. In 1927, GE released the Monitor Top, the first refrigerator to run on electricity.
In 1930, Frigidaire, one of GE's main competitors, synthesized 
 The vapor-compression cycle is used in most household refrigerators as well as in many large commercial and industrial refrigeration systems. Figure 1 provides a schematic diagram of the components of a typical vapor-compression refrigeration system.
The
The vapor-compression cycle is used in most household refrigerators as well as in many large commercial and industrial refrigeration systems. Figure 1 provides a schematic diagram of the components of a typical vapor-compression refrigeration system.
The