|
Dilution Refrigerator
A 3He/4He dilution refrigerator is a cryogenics, cryogenic device that provides continuous cooling to temperatures as low as 2 Kelvin, mK, with no moving parts in the low-temperature region. The cooling power is provided by the heat of mixing of the Helium-3 and Helium-4 isotopes. The dilution refrigerator was first proposed by Heinz London in the early 1950s, and was experimentally realized in 1964 in the Kamerlingh Onnes Laboratorium at Leiden University. The field of dilution refrigeration is reviewed by Zu et al. Theory of operation The refrigeration process uses a mixture of two isotopes of helium: helium-3 and helium-4. When cooled below approximately Orders of magnitude (temperature), 870 Kelvin, millikelvins, the mixture undergoes spontaneous phase separation to form a 3He-rich phase (the concentrated phase) and a 3He-poor phase (the dilute phase). As shown in the phase diagram, at very low temperatures the concentrated phase is essentially pure 3He, whil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Phase Diagram
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol (chemistry), symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the Chemical element, elements. It is the second lightest and second most Abundance of the chemical elements, abundant element in the observable universe (hydrogen is the lightest and most abundant). It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and in Jupiter, due to the very high nuclear binding energy (per nucleon) of helium-4, with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth, produced after the planet cooled and solidified. While it is also produced by nuclear fusion in stars, most helium-4 in the Sun and in the universe is thought to have been produced by the Big Bang, and is referred to as " primordial helium". However, primordial helium-4 is largely absent from the Earth, having escaped during the high-temperature phase of Earth's formation. Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen. When liquid helium-4 is cooled to below , it becomes a superfluid, with properties that are ver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Demagnetization
Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures, as well as the ranges used in common refrigerators. A magnetocaloric material warms up when a magnetic field is applied. The warming is due to changes in the internal state of the material releasing heat. When the magnetic field is removed, the material returns to its original state, reabsorbing the heat, and returning to original temperature. To achieve refrigeration, the material is allowed to radiate away its heat while in the magnetized hot state. Removing the magnetism, the material then cools to ''below'' its original temperature. The effect was first observed in 1881 by a German physicist Emil Warburg, followed by French physicist P. Weiss and Swiss physicist A. Piccard in 1917. The fundamental principle was suggested by P. Debye (1926) and W. Giauque (1927). The first working magnetic refrigerators were constructed by s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Refrigeration
Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This technique can be used to attain extremely low temperatures, as well as the ranges used in common refrigerators. A magnetocaloric material warms up when a magnetic field is applied. The warming is due to changes in the internal state of the material releasing heat. When the magnetic field is removed, the material returns to its original state, reabsorbing the heat, and returning to original temperature. To achieve refrigeration, the material is allowed to radiate away its heat while in the magnetized hot state. Removing the magnetism, the material then cools to ''below'' its original temperature. The effect was first observed in 1881 by a German physicist Emil Warburg, followed by French physicist P. Weiss and Swiss physicist A. Piccard in 1917. The fundamental principle was suggested by P. Debye (1926) and W. Giauque (1927). The first working magnetic refrigerators were constructed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kapitza Resistance
Interfacial thermal resistance, also known as thermal boundary resistance, or Kapitza resistance, is a measure of resistance to thermal flow at the interface between two materials. While these terms may be used interchangeably, Kapitza resistance technically refers to an atomically perfect, flat interface whereas thermal boundary resistance is a more broad term. This thermal resistance differs from contact resistance (not to be confused with electrical contact resistance) because it exists even at atomically perfect interfaces. Owing to differences in electronic and vibrational properties in different materials, when an energy carrier (phonon or electron, depending on the material) attempts to traverse the interface, it will scatter at the interface. The probability of transmission after scattering will depend on the available energy states on side 1 and side 2 of the interface. Assuming a constant thermal flux is applied across an interface, this interfacial thermal resistance wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulse Tube Refrigerator
The pulse tube refrigerator (PTR) or pulse tube cryocooler is a developing technology that emerged largely in the early 1980s with a series of other innovations in the broader field of thermoacoustics. In contrast with other cryocoolers (e.g. applications of the Stirling engine#Stirling cryocoolers, Stirling cryocooler and cryocooler#GM-refrigerators, GM-refrigerators), this cryocooler can be made without moving parts in the low temperature part of the device, making the cooler suitable for a wide variety of applications. Uses Pulse tube cryocoolers are used in industrial applications such as semiconductor fabrication and in military applications such as for the cooling of infrared sensors. Pulse tubes are also being developed for cooling of astronomical detectors where liquid cryogens are typically used, such as the Atacama Cosmology Telescope or the Qubic experiment (an interferometer for cosmology studies). PTRs are used as precoolers of dilution refrigerators. Pulse tubes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Helium
Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity. At standard pressure, the chemical element helium exists in a liquid form only at the extremely low temperature of . Its boiling point and critical point depend on which isotope of helium is present: the common isotope helium-4 or the rare isotope helium-3. These are the only two stable isotopes of helium. See the table below for the values of these physical quantities. The density of liquid helium-4 at its boiling point and a pressure of one atmosphere (101.3 kilopascals) is about , or about one-eighth the density of liquid water. Liquefaction Helium was first liquefied on July 10, 1908, by the Dutch physicist Heike Kamerlingh Onnes at the University of Leiden in the Netherlands. At that time, helium-3 was unknown because the mass spectrometer had not yet been invented. In more recent decades, liquid helium has been used as a cryogenic ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulse Tube Refrigerator
The pulse tube refrigerator (PTR) or pulse tube cryocooler is a developing technology that emerged largely in the early 1980s with a series of other innovations in the broader field of thermoacoustics. In contrast with other cryocoolers (e.g. applications of the Stirling engine#Stirling cryocoolers, Stirling cryocooler and cryocooler#GM-refrigerators, GM-refrigerators), this cryocooler can be made without moving parts in the low temperature part of the device, making the cooler suitable for a wide variety of applications. Uses Pulse tube cryocoolers are used in industrial applications such as semiconductor fabrication and in military applications such as for the cooling of infrared sensors. Pulse tubes are also being developed for cooling of astronomical detectors where liquid cryogens are typically used, such as the Atacama Cosmology Telescope or the Qubic experiment (an interferometer for cosmology studies). PTRs are used as precoolers of dilution refrigerators. Pulse tubes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
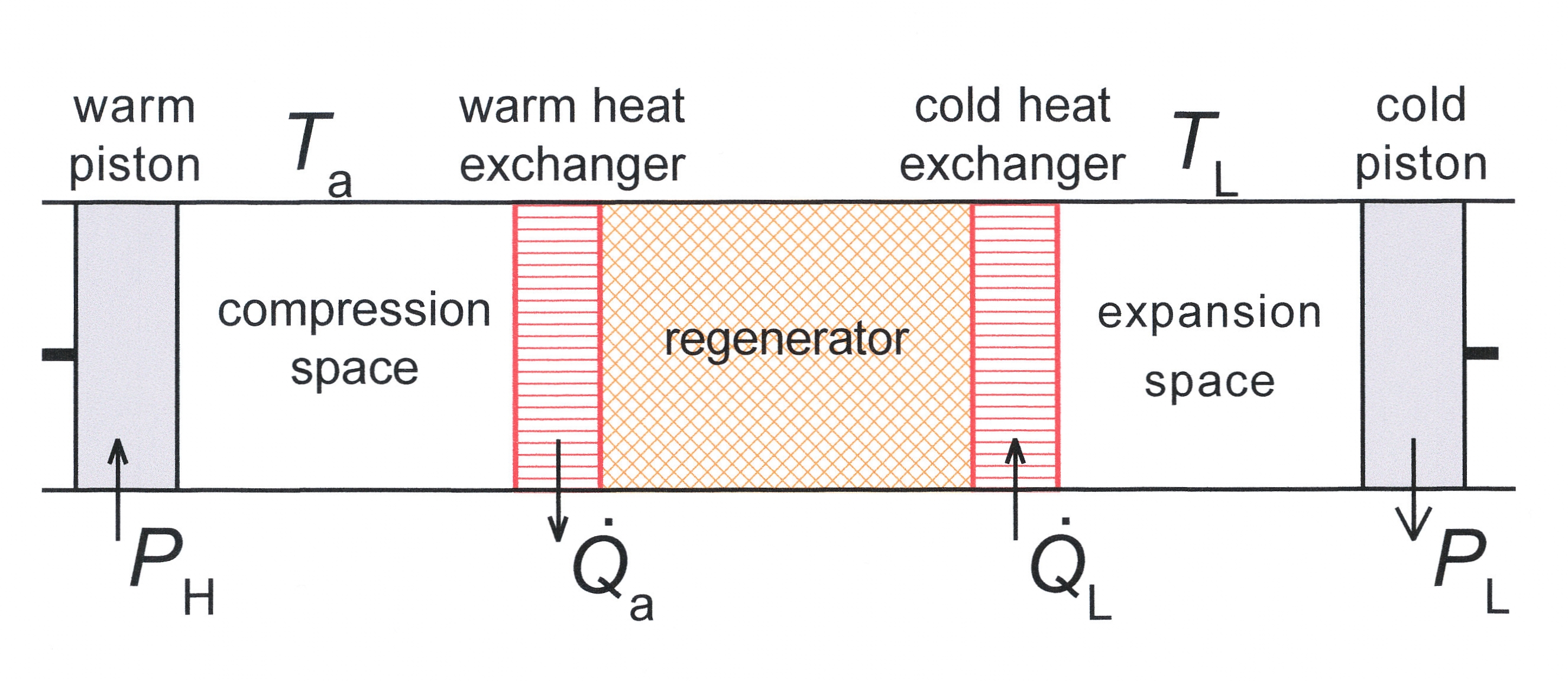
Cryocooler
A refrigerator designed to reach cryogenic temperatures (below ) is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as low as 2–3 W. Large systems, such as those used for cooling the superconducting magnets in particle accelerators are more often called cryogenic refrigerators. Their input powers can be as high as 1 MW. In most cases cryocoolers use a cryogenic fluid as the working substance and employ moving parts to cycle the fluid around a thermodynamic cycle. The fluid is typically compressed at room temperature, precooled in a heat exchanger, then expanded at some low temperature. The returning low-pressure fluid passes through the heat exchanger to precool the high-pressure fluid before entering the compressor intake. The cycle is then repeated. __TOC__ Ideal heat exchangers and regenerators Heat exchangers are important components of all cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superfluid
Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortices that continue to rotate indefinitely. Superfluidity occurs in two isotopes of helium (helium-3 and helium-4) when they are liquefied by cooling to cryogenic temperatures. It is also a property of various other exotic states of matter theorized to exist in astrophysics, high-energy physics, and theories of quantum gravity. The theory of superfluidity was developed by Soviet theoretical physicists Lev Landau and Isaak Khalatnikov. Superfluidity is often coincidental with Bose–Einstein condensation, but neither phenomenon is directly related to the other; not all Bose–Einstein condensates can be regarded as superfluids, and not all superfluids are Bose–Einstein condensates. Superfluidity of liquid helium Superfluidity was discovered in helium-4 by Pyotr Kapitsa and independently by John F. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Condensation
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. The enthalpy of vaporization is a function of the pressure at which that transformation takes place. The enthalpy of vaporization is often quoted for the normal boiling temperature of the substance. Although tabulated values are usually corrected to 298 K, that correction is often smaller than the uncertainty in the measured value. The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and for reduced temperature T_r \ll 1. The heat of vaporization diminishes with increasing temperature and it vanishes completely at a certain point called the critical temperature (T_r = 1). Above the critical temperature, the liquid and vapor phases are indistinguishable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is widely used as a coolant. Physical properties The diatomic character of the N2 molecule is retained after liquefaction. The weak van der Waals interaction between the N2 molecules results in little interatomic interaction, manifested in its very low boiling point. The temperature of liquid nitrogen can readily be reduced to its freezing point by placing it in a vacuum chamber pumped by a vacuum pump. Liquid nitrogen's efficiency as a coolant is limited by the fact that it boils immediately on contact with a warmer object, enveloping the object in an insulating layer of nitrogen gas bubbles. This effect, known as the Leidenfrost effect, occurs when any liquid comes in contact with a surface which is significantly hotter than its boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |