Caesium chloride.jpg on:
[Wikipedia]
[Google]
[Amazon]
Caesium ( IUPAC spelling) (or cesium in
 Of all elements that are solid at room temperature, caesium is the softest: it has a hardness of 0.2 Mohs. It is a very ductile, pale metal, which darkens in the presence of trace amounts of oxygen. When in the presence of mineral oil (where it is best kept during transport), it loses its metallic
Of all elements that are solid at room temperature, caesium is the softest: it has a hardness of 0.2 Mohs. It is a very ductile, pale metal, which darkens in the presence of trace amounts of oxygen. When in the presence of mineral oil (where it is best kept during transport), it loses its metallic  Caesium forms alloys with the other alkali metals, gold, and mercury (
Caesium forms alloys with the other alkali metals, gold, and mercury (
 Most caesium compounds contain the element as the
Most caesium compounds contain the element as the

 More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
 The radioactive 135Cs has a very long half-life of about 2.3 million years, the longest of all radioactive isotopes of caesium. 137Cs and 134Cs have half-lives of 30 and two years, respectively. 137Cs decomposes to a short-lived 137mBa by beta decay, and then to nonradioactive barium, while 134Cs transforms into 134Ba directly. The isotopes with mass numbers of 129, 131, 132 and 136, have half-lives between a day and two weeks, while most of the other isotopes have half-lives from a few seconds to fractions of a second. At least 21 metastable nuclear isomers exist. Other than 134mCs (with a half-life of just under 3 hours), all are very unstable and decay with half-lives of a few minutes or less.
The isotope 135Cs is one of the
The radioactive 135Cs has a very long half-life of about 2.3 million years, the longest of all radioactive isotopes of caesium. 137Cs and 134Cs have half-lives of 30 and two years, respectively. 137Cs decomposes to a short-lived 137mBa by beta decay, and then to nonradioactive barium, while 134Cs transforms into 134Ba directly. The isotopes with mass numbers of 129, 131, 132 and 136, have half-lives between a day and two weeks, while most of the other isotopes have half-lives from a few seconds to fractions of a second. At least 21 metastable nuclear isomers exist. Other than 134mCs (with a half-life of just under 3 hours), all are very unstable and decay with half-lives of a few minutes or less.
The isotope 135Cs is one of the
 Caesium is a relatively rare element, estimated to average 3 parts per million in the
Caesium is a relatively rare element, estimated to average 3 parts per million in the
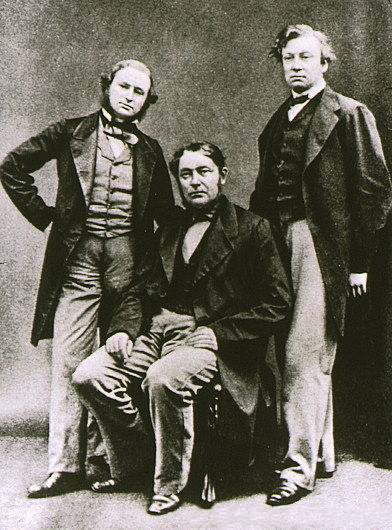 In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Dürkheim, Germany. Because of the bright blue lines in the
In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Dürkheim, Germany. Because of the bright blue lines in the
 Caesium-based atomic clocks use the electromagnetic transitions in the
Caesium-based atomic clocks use the electromagnetic transitions in the
 Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as
Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as
 Caesium and mercury were used as a propellant in early
Caesium and mercury were used as a propellant in early
 Nonradioactive caesium compounds are only mildly toxic, and nonradioactive caesium is not a significant environmental hazard. Because biochemical processes can confuse and substitute caesium with potassium, excess caesium can lead to hypokalemia,
Nonradioactive caesium compounds are only mildly toxic, and nonradioactive caesium is not a significant environmental hazard. Because biochemical processes can confuse and substitute caesium with potassium, excess caesium can lead to hypokalemia,
Caesium or Cesium
at '' The Periodic Table of Videos'' (University of Nottingham)
View the reaction of Caesium (most reactive metal in the periodic table) with Fluorine (most reactive non-metal)
courtesy of The Royal Institution. * {{Authority control Alkali metals Chemical elements with body-centered cubic structure Chemical elements Glycine receptor agonists Reducing agents Articles containing video clips
American English
American English, sometimes called United States English or U.S. English, is the set of variety (linguistics), varieties of the English language native to the United States. English is the Languages of the United States, most widely spoken lan ...
) is a chemical element with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Cs and atomic number 55. It is a soft, silvery-golden alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
with a melting point of , which makes it one of only five elemental metals that are liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
at or near room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Caesium has physical and chemical properties similar to those of rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
. It has only one stable isotope, caesium-133
Caesium (55Cs) has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. The atomic masses of these isotopes range from 112 to 151. Only one isotope, 133Cs, is stable. The longest-lived radioisoto ...
. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
and mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, one of the elements with the most isotopes. Caesium-137, a fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
, is extracted from waste produced by nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
.
The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy
A flame (from Latin ''wikt:en:flamma#Latin, flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of suf ...
. The first small-scale applications for caesium were as a " getter" in vacuum tubes and in photoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
. In 1967, acting on Einstein's proof that the speed of light is the most-constant dimension in the universe, the International System of Units
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. E ...
used two specific wave counts from an emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
of caesium-133 to co-define the second
The second (symbol: s) is the unit of time in the International System of Units (SI), historically defined as of a day – this factor derived from the division of the day first into 24 hours, then to 60 minutes and finally to 60 seconds ...
and the metre. Since then, caesium has been widely used in highly accurate atomic clocks.
Since the 1990s, the largest application of the element has been as caesium formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''U ...
for drilling fluids, but it has a range of applications in the production of electricity, in electronics, and in chemistry. The radioactive isotope caesium-137 has a half-life of about 30 years and is used in medical applications, industrial gauges, and hydrology. Nonradioactive caesium compounds are only mildly toxic, but the pure metal's tendency to react explosively with water means that caesium is considered a hazardous material, and the radioisotopes present a significant health and ecological hazard in the environment.
Characteristics
Physical properties
lustre
Lustre or Luster may refer to:
Places
* Luster, Norway, a municipality in Vestlandet, Norway
** Luster (village), a village in the municipality of Luster
* Lustre, Montana, an unincorporated community in the United States
Entertainment
* '' ...
and takes on a duller, grey appearance. It has a melting point of , making it one of the few elemental metals that are liquid near room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
is the only stable elemental metal with a known melting point lower than caesium. In addition, the metal has a rather low boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
, , the lowest
Low or LOW or lows, may refer to:
People
* Low (surname), listing people surnamed Low
Places
* Low, Quebec, Canada
* Low, Utah, United States
* Lo Wu station (MTR code LOW), Hong Kong; a rail station
* Salzburg Airport (ICAO airport code: LOW ...
of all metals other than mercury. Its compounds burn with a blue or violet colour.
 Caesium forms alloys with the other alkali metals, gold, and mercury (
Caesium forms alloys with the other alkali metals, gold, and mercury (amalgams
Amalgam most commonly refers to:
* Amalgam (chemistry), mercury alloy
* Amalgam (dentistry), material of silver tooth fillings
** Bonded amalgam, used in dentistry
Amalgam may also refer to:
* Amalgam Comics, a publisher
* Amalgam Digital, an in ...
). At temperatures below , it does not alloy with cobalt, iron, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
, nickel, platinum, tantalum, or tungsten. It forms well-defined intermetallic compounds with antimony, gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, indium, and thorium, which are photosensitive. It mixes with all the other alkali metals (except lithium); the alloy with a molar distribution of 41% caesium, 47% potassium, and 12% sodium has the lowest melting point of any known metal alloy, at . A few amalgams have been studied: is black with a purple metallic lustre
Lustre or Luster may refer to:
Places
* Luster, Norway, a municipality in Vestlandet, Norway
** Luster (village), a village in the municipality of Luster
* Lustre, Montana, an unincorporated community in the United States
Entertainment
* '' ...
, while CsHg is golden-coloured, also with a metallic lustre.
The golden colour of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended. For lithium through rubidium this frequency is in the ultraviolet, but for caesium it enters the blue–violet end of the spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium. Thus caesium transmits and partially absorbs violet light preferentially while other colours (having lower frequency) are reflected; hence it appears yellowish.
Chemical properties
Caesium metal is highly reactive and very pyrophoric. It ignites spontaneously in air, and reacts explosively with water even at low temperatures, more so than the other alkali metals (first group
FirstGroup plc is a British multi-national transport group, based in Aberdeen, Scotland.periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
). It reacts with ice at temperatures as low as . Because of this high reactivity, caesium metal is classified as a hazardous material
Dangerous goods, abbreviated DG, are substances that when transported are a risk to health, safety, property or the environment. Certain dangerous goods that pose risks even when not being transported are known as hazardous materials ( syllabi ...
. It is stored and shipped in dry, saturated hydrocarbons such as mineral oil. It can be handled only under inert gas, such as argon. However, a caesium-water explosion is often less powerful than a sodium-water explosion with a similar amount of sodium. This is because caesium explodes instantly upon contact with water, leaving little time for hydrogen to accumulate. Caesium can be stored in vacuum-sealed borosilicate glass ampoules. In quantities of more than about , caesium is shipped in hermetically sealed, stainless steel containers.
The chemistry of caesium is similar to that of other alkali metals, in particular rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
, the element above caesium in the periodic table. As expected for an alkali metal, the only common oxidation state is +1. Some slight differences arise from the fact that it has a higher atomic mass and is more electropositive than other (nonradioactive) alkali metals. Caesium is the most electropositive chemical element. The caesium ion is also larger and less "hard" than those of the lighter alkali metals.
Compounds
 Most caesium compounds contain the element as the
Most caesium compounds contain the element as the cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, which binds ionically to a wide variety of anions. One noteworthy exception is the caeside anion (), and others are the several suboxides (see section on oxides below). More recently, caesium is predicted to behave as a p-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
element and capable of forming higher fluorides with higher oxidation states (i.e., CsFn with n > 1) under high pressure. This prediction needs to be validated by further experiments.
Salts of Cs+ are usually colourless unless the anion itself is coloured. Many of the simple salts are hygroscopic, but less so than the corresponding salts of lighter alkali metals. The phosphate, acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
, carbonate, halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s, oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
, nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
, and sulfate salts are water-soluble. Its double salt
A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula ) and Tutton's salts (with the general formula ). Other examples include potassium sodium tartrate, ammo ...
s are often less soluble, and the low solubility of caesium aluminium sulfate is exploited in refining Cs from ores. The double salts with antimony (such as ), bismuth, cadmium, copper, iron, and lead are also poorly soluble.
Caesium hydroxide (CsOH) is hygroscopic and strongly basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
. It rapidly etches the surface of semiconductors such as silicon. CsOH has been previously regarded by chemists as the "strongest base", reflecting the relatively weak attraction between the large Cs+ ion and OH−; it is indeed the strongest Arrhenius base Arrhenius may refer to
* Birgit Arrhenius (born 1932), Swedish archaeologist
* Carl Axel Arrhenius (1757–1824), Swedish army lieutenant and amateur mineralogist who discovered ytterbite, a mineral that led to the discovery of yttrium by Johan Gad ...
; however, a number of compounds such as ''n''-butyllithium, sodium amide, sodium hydride, caesium hydride, etc., which cannot be dissolved in water as reacting violently with it but rather only used in some anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
polar aprotic solvents A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
, are far more basic on the basis of the Brønsted–Lowry acid–base theory.
A stoichiometric mixture of caesium and gold will react to form yellow caesium auride
Caesium auride is the inorganic compound with the formula CsAu. It is the Cs+ salt of the unusual Au− anion.
Preparation and reactions
CsAu is obtained by heating a Stoichiometry, stoichiometric mixture of caesium and gold. The two metallic-yel ...
(Cs+Au−) upon heating. The auride anion here behaves as a pseudohalogen. The compound reacts violently with water, yielding caesium hydroxide, metallic gold, and hydrogen gas; in liquid ammonia it can be reacted with a caesium-specific ion exchange resin to produce tetramethylammonium auride
Tetramethylammonium auride, , is an ionic compound containing tetramethylammonium as cation and gold in a –1 oxidation state as anion. It is an example of a compound containing this rare ionic form of gold, and the first auride paired with a ca ...
. The analogous platinum compound, red caesium platinide (Cs2Pt), contains the platinide ion that behaves as a pseudo chalcogen.
Complexes
Like all metal cations, Cs+ forms complexes withLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s in solution. Because of its large size, Cs+ usually adopts coordination numbers greater than 6, the number typical for the smaller alkali metal cations. This difference is apparent in the 8-coordination of CsCl. This high coordination number and softness (tendency to form covalent bonds) are properties exploited in separating Cs+ from other cations in the remediation of nuclear wastes, where 137Cs+ must be separated from large amounts of nonradioactive K+.
Halides

Caesium fluoride
Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electroposit ...
(CsF) is a hygroscopic white solid that is widely used in organofluorine chemistry
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents ...
as a source of fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
anions. Caesium fluoride has the halite structure, which means that the Cs+ and F− pack in a cubic closest packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
array as do Na+ and Cl− in sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. Notably, caesium and fluorine have the lowest and highest electronegativities
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
, respectively, among all the known elements.
Caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
(CsCl) crystallizes in the simple cubic crystal system
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
. Also called the "caesium chloride structure", this structural motif is composed of a primitive cubic lattice with a two-atom basis, each with an eightfold coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a centr ...
; the chloride atoms lie upon the lattice points at the edges of the cube, while the caesium atoms lie in the holes in the centre of the cubes. This structure is shared with CsBr and CsI, and many other compounds that do not contain Cs. In contrast, most other alkaline halides have the sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(NaCl) structure. The CsCl structure is preferred because Cs+ has an ionic radius of 174 pm and 181 pm.
Oxides
 More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with
More so than the other alkali metals, caesium forms numerous binary compounds with oxygen. When caesium burns in air, the superoxide is the main product. The "normal" caesium oxide () forms yellow-orange hexagonal crystals, and is the only oxide of the anti- type. It vaporizes at , and decomposes to caesium metal and the peroxide at temperatures above . In addition to the superoxide and the ozonide , several brightly coloured suboxides have also been studied. These include , , , (dark-green), CsO, , as well as . The latter may be heated in a vacuum to generate . Binary compounds with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, selenium, and tellurium also exist.
Isotopes
Caesium has 40 known isotopes, ranging in mass number (i.e. number of nucleons in the nucleus) from 112 to 151. Several of these are synthesized from lighter elements by the slow neutron capture process (S-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
) inside old stars and by the R-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
in supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
explosions. The only stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
caesium isotope is 133Cs, with 78 neutrons. Although it has a large nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe th ...
(+), nuclear magnetic resonance studies can use this isotope at a resonating frequency of 11.7 MHz.
long-lived fission product
Long-lived fission products (LLFPs) are radioactive materials with a long half-life (more than 200,000 years) produced by nuclear fission of uranium and plutonium. Because of their persistent radiotoxicity it is necessary to isolate them from man ...
s of uranium produced in nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
. However, this fission product yield is reduced in most reactors because the predecessor, 135Xe, is a potent neutron poison and frequently transmutes to stable 136Xe before it can decay to 135Cs.
The beta decay from 137Cs to 137mBa is a strong emission of gamma radiation
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically s ...
. 137Cs and 90Sr are the principal medium-lived products of nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
, and the prime sources of radioactivity from spent nuclear fuel after several years of cooling, lasting several hundred years. Those two isotopes are the largest source of residual radioactivity in the area of the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
. Because of the low capture rate, disposing of 137Cs through neutron capture is not feasible and the only current solution is to allow it to decay over time.
Almost all caesium produced from nuclear fission comes from the beta decay of originally more neutron-rich fission products, passing through various isotopes of iodine and xenon. Because iodine and xenon are volatile and can diffuse through nuclear fuel or air, radioactive caesium is often created far from the original site of fission. With nuclear weapons testing in the 1950s through the 1980s, 137Cs was released into the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
and returned to the surface of the earth as a component of radioactive fallout. It is a ready marker of the movement of soil and sediment from those times.
Occurrence
 Caesium is a relatively rare element, estimated to average 3 parts per million in the
Caesium is a relatively rare element, estimated to average 3 parts per million in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
. It is the 45th most abundant element and the 36th among the metals. Nevertheless, it is more abundant than such elements as antimony, cadmium, tin, and tungsten, and two orders of magnitude more abundant than mercury and silver; it is 3.3% as abundant as rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
, with which it is closely associated, chemically.
Due to its large ionic radius, caesium is one of the " incompatible elements". During magma crystallization
Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natur ...
, caesium is concentrated in the liquid phase and crystallizes last. Therefore, the largest deposits of caesium are zone pegmatite
A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than and sometimes greater than . Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic com ...
ore bodies formed by this enrichment process. Because caesium does not substitute for potassium as readily as rubidium does, the alkali evaporite minerals sylvite
Sylvite, or sylvine, is potassium chloride (KCl) in natural mineral form. It forms crystals in the isometric system very similar to normal rock salt, halite ( NaCl). The two are, in fact, isomorphous. Sylvite is colorless to white with shades of ...
(KCl) and carnallite () may contain only 0.002% caesium. Consequently, caesium is found in few minerals. Percentage amounts of caesium may be found in beryl () and avogadrite
Avogadrite ((K,Cs)BF4) is a potassium- caesium tetrafluoroborate in the halide class. Avogadrite crystallizes in the orthorhombic system (space group ''Pnma'') with cell parameters ''a'' 8.66 Å, ''b'' 5.48 Å and ''c'' Å 7.03.
History
The min ...
(), up to 15 wt% Cs2O in the closely related mineral pezzottaite
Pezzottaite, marketed under the name raspberyl or raspberry beryl, is a mineral species first recognized by the International Mineralogical Association in September 2003. Pezzottaite is a caesium analogue of beryl, a silicate of caesium, beryll ...
(), up to 8.4 wt% Cs2O in the rare mineral londonite
The borate minerals are minerals which contain a borate anion group. The borate (BO3) units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures whic ...
(), and less in the more widespread rhodizite. The only economically important ore for caesium is pollucite , which is found in a few places around the world in zoned pegmatites, associated with the more commercially important lithium minerals, lepidolite and petalite. Within the pegmatites, the large grain size and the strong separation of the minerals results in high-grade ore for mining.
The world's most significant and richest known source of caesium is the Tanco Mine at Bernic Lake in Manitoba, Canada, estimated to contain 350,000 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United States ...
of pollucite ore, representing more than two-thirds of the world's reserve base. Although the stoichiometric content of caesium in pollucite is 42.6%, pure pollucite samples from this deposit contain only about 34% caesium, while the average content is 24 wt%. Commercial pollucite contains more than 19% caesium. The Bikita pegmatite deposit in Zimbabwe is mined for its petalite, but it also contains a significant amount of pollucite. Another notable source of pollucite is in the Karibib Desert, Namibia. At the present rate of world mine production of 5 to 10 metric tons per year, reserves will last for thousands of years.
Production
Mining and refining pollucite ore is a selective process and is conducted on a smaller scale than for most other metals. The ore is crushed, hand-sorted, but not usually concentrated, and then ground. Caesium is then extracted from pollucite primarily by three methods: acid digestion, alkaline decomposition, and direct reduction. In the acid digestion, thesilicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is al ...
pollucite rock is dissolved with strong acids, such as hydrochloric (HCl), sulfuric
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
(), hydrobromic (HBr), or hydrofluoric (HF) acids. With hydrochloric acid, a mixture of soluble chlorides is produced, and the insoluble chloride double salts of caesium are precipitated as caesium antimony chloride (), caesium iodine chloride (), or caesium hexachlorocerate (). After separation, the pure precipitated double salt is decomposed, and pure CsCl is precipitated by evaporating the water.
The sulfuric acid method yields the insoluble double salt directly as caesium alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , where is a valence (chemistry), monovalent cation such as potassium or a ...
(). The aluminium sulfate
Aluminium sulfate is a salt with the chemical formula, formula aluminium, Al2sulfate, (SO4)3. It is soluble in water and is mainly used as a Coagulation (water treatment), coagulating agent (promoting particle collision by neutralizing charge) in ...
component is converted to insoluble aluminium oxide by roasting the alum with carbon, and the resulting product is leached with water to yield a solution.
Roasting pollucite with calcium carbonate and calcium chloride yields insoluble calcium silicates and soluble caesium chloride. Leaching with water or dilute ammonia () yields a dilute chloride (CsCl) solution. This solution can be evaporated to produce caesium chloride or transformed into caesium alum or caesium carbonate. Though not commercially feasible, the ore can be directly reduced with potassium, sodium, or calcium in vacuum to produce caesium metal directly.
Most of the mined caesium (as salts) is directly converted into caesium formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''U ...
(HCOO−Cs+) for applications such as oil drilling. To supply the developing market, Cabot Corporation built a production plant in 1997 at the Tanco mine near Bernic Lake in Manitoba, with a capacity of per year of caesium formate solution. The primary smaller-scale commercial compounds of caesium are caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
and nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
.
Alternatively, caesium metal may be obtained from the purified compounds derived from the ore. Caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
and the other caesium halides can be reduced at with calcium or barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, and caesium metal distilled from the result. In the same way, the aluminate, carbonate, or hydroxide may be reduced by magnesium.
The metal can also be isolated by electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of fused caesium cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
(CsCN). Exceptionally pure and gas-free caesium can be produced by thermal decomposition of caesium azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
, which can be produced from aqueous caesium sulfate and barium azide
Barium azide is an inorganic azide with the formula . It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.
Preparation
Barium azide may be prepared by reacting sodiu ...
. In vacuum applications, caesium dichromate can be reacted with zirconium to produce pure caesium metal without other gaseous products.
: + 2 → 2 + 2 +
The price of 99.8% pure caesium (metal basis) in 2009 was about , but the compounds are significantly cheaper.
History
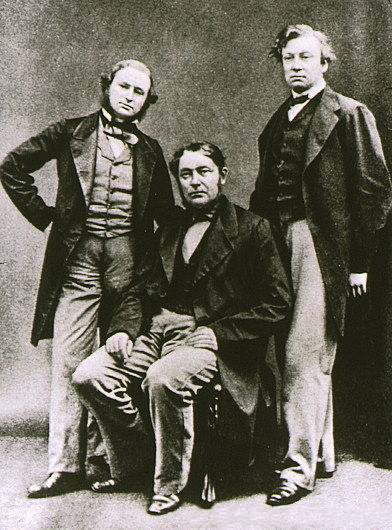 In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Dürkheim, Germany. Because of the bright blue lines in the
In 1860, Robert Bunsen and Gustav Kirchhoff discovered caesium in the mineral water from Dürkheim, Germany. Because of the bright blue lines in the emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
, they derived the name from the Latin word ''caesius'', meaning sky-blue.Bunsen quotes Aulus Gellius Noctes Atticae II, 26 by Nigidius Figulus
Publius Nigidius Figulus (c. 98 – 45 BC) was a scholar of the Late Roman Republic and one of the praetors for 58 BC. He was a friend of Cicero, to whom he gave his support at the time of the Catilinarian conspiracy. Nigidius sided with the Optim ...
: ''Nostris autem veteribus caesia dicts est quae Graecis, ut Nigidus ait, de colore coeli quasi coelia.'' Caesium was the first element to be discovered with a spectroscope, which had been invented by Bunsen and Kirchhoff only a year previously.
To obtain a pure sample of caesium, of mineral water had to be evaporated to yield of concentrated salt solution. The alkaline earth metals were precipitated either as sulfates or oxalates, leaving the alkali metal in the solution. After conversion to the nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
s and extraction with ethanol, a sodium-free mixture was obtained. From this mixture, the lithium was precipitated by ammonium carbonate. Potassium, rubidium, and caesium form insoluble salts with chloroplatinic acid
Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula 3Osub>2 tCl6H2O)''x'' (0 ≤ ''x'' ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Alth ...
, but these salts show a slight difference in solubility in hot water, and the less-soluble caesium and rubidium hexachloroplatinate ((Cs,Rb)2PtCl6) were obtained by fractional crystallization Fractional crystallization may refer to:
* Fractional crystallization (chemistry), a process to separate different solutes from a solution
* Fractional crystallization (geology)
Fractional crystallization, or crystal fractionation, is one of the ...
. After reduction of the hexachloroplatinate with hydrogen, caesium and rubidium were separated by the difference in solubility of their carbonates in alcohol. The process yielded of rubidium chloride and of caesium chloride from the initial 44,000 litres of mineral water.
From the caesium chloride, the two scientists estimated the atomic weight of the new element at 123.35 (compared to the currently accepted one of 132.9). They tried to generate elemental caesium by electrolysis of molten caesium chloride, but instead of a metal, they obtained a blue homogeneous substance which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance"; as a result, they assigned it as a subchloride (). In reality, the product was probably a colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
al mixture of the metal and caesium chloride. The electrolysis of the aqueous solution of chloride with a mercury cathode produced a caesium amalgam which readily decomposed under the aqueous conditions. The pure metal was eventually isolated by the Swedish chemist Carl Setterberg ''Carl'' Theodor Setterberg, (born 30. April 1853 in Järpås socken, dead 7. April 1941 in Stockholm
Stockholm () is the Capital city, capital and List of urban areas in Sweden by population, largest city of Sweden as well as the List of u ...
while working on his doctorate with Kekulé and Bunsen. In 1882, he produced caesium metal by electrolysing caesium cyanide
Caesium cyanide (chemical formula: CsCN) is the caesium salt of hydrogen cyanide. It is a white solid, easily soluble in water, with a smell reminiscent of bitter almonds, and with crystals similar in appearance to sugar. Caesium cyanide has chemic ...
, avoiding the problems with the chloride.
Historically, the most important use for caesium has been in research and development, primarily in chemical and electrical fields. Very few applications existed for caesium until the 1920s, when it came into use in radio vacuum tubes, where it had two functions; as a getter, it removed excess oxygen after manufacture, and as a coating on the heated cathode, it increased the electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
. Caesium was not recognized as a high-performance industrial metal until the 1950s. Applications for nonradioactive caesium included photoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
, photomultiplier tubes, optical components of infrared spectrophotometers, catalysts for several organic reactions, crystals for scintillation counter
A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.
It consists of a scintillator w ...
s, and in magnetohydrodynamic power generators. Caesium is also used as a source of positive ions in secondary ion mass spectrometry (SIMS).
Since 1967, the International System of Measurements has based the primary unit of time, the second, on the properties of caesium. The International System of Units (SI) defines the second as the duration of 9,192,631,770 cycles at the microwave frequency of the spectral line corresponding to the transition between two hyperfine
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nuc ...
energy levels of the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
of caesium-133
Caesium (55Cs) has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. The atomic masses of these isotopes range from 112 to 151. Only one isotope, 133Cs, is stable. The longest-lived radioisoto ...
. The 13th General Conference on Weights and Measures
The General Conference on Weights and Measures (GCWM; french: Conférence générale des poids et mesures, CGPM) is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established i ...
of 1967 defined a second as: "the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground state undisturbed by external fields".
Applications
Petroleum exploration
The largest present-day use of nonradioactive caesium is incaesium formate
Formate (IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ''U ...
drilling fluids for the extractive oil industry
The petroleum industry, also known as the oil industry or the oil patch, includes the global processes of exploration, extraction, refining, transportation (often by oil tankers and pipelines), and marketing of petroleum products. The largest ...
. Aqueous solutions of caesium formate (HCOO−Cs+)—made by reacting caesium hydroxide with formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
—were developed in the mid-1990s for use as oil well drilling and completion fluids. The function of a drilling fluid is to lubricate drill bits, to bring rock cuttings to the surface, and to maintain pressure on the formation during drilling of the well. Completion fluids assist the emplacement of control hardware after drilling but prior to production by maintaining the pressure.
The high density of the caesium formate brine (up to 2.3 g/cm3, or 19.2 pounds per gallon), coupled with the relatively benign nature of most caesium compounds, reduces the requirement for toxic high-density suspended solids in the drilling fluid—a significant technological, engineering and environmental advantage. Unlike the components of many other heavy liquids, caesium formate is relatively environment-friendly. Caesium formate brine can be blended with potassium and sodium formates to decrease the density of the fluids to that of water (1.0 g/cm3, or 8.3 pounds per gallon). Furthermore, it is biodegradable and may be recycled, which is important in view of its high cost (about $4,000 per barrel
A barrel or cask is a hollow cylindrical container with a bulging center, longer than it is wide. They are traditionally made of wooden staves and bound by wooden or metal hoops. The word vat is often used for large containers for liquids, ...
in 2001). Alkali formates are safe to handle and do not damage the producing formation or downhole metals as corrosive alternative, high-density brines (such as zinc bromide solutions) sometimes do; they also require less cleanup and reduce disposal costs.
Atomic clocks
 Caesium-based atomic clocks use the electromagnetic transitions in the
Caesium-based atomic clocks use the electromagnetic transitions in the hyperfine structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucl ...
of caesium-133 atoms as a reference point. The first accurate caesium clock was built by Louis Essen
Louis Essen FRS O.B.E. (6 September 1908 – 24 August 1997) was an English physicist whose most notable achievements were in the precise measurement of time and the determination of the speed of light. He was a critic of Albert Einstein' ...
in 1955 at the National Physical Laboratory in the UK. Caesium clocks have improved over the past half-century and are regarded as "the most accurate realization of a unit that mankind has yet achieved." These clocks measure frequency with an error of 2 to 3 parts in 1014, which corresponds to an accuracy of 2 nanoseconds per day, or one second in 1.4 million years. The latest versions are more accurate than 1 part in 1015, about 1 second in 20 million years. The caesium standard
The caesium standard is a primary frequency standard in which the Absorption (electromagnetic radiation), photon absorption by transitions between the two hyperfine level, hyperfine ground states of caesium-133 atoms is used to control the output ...
is the primary standard for standards-compliant time and frequency measurements. Caesium clocks regulate the timing of cell phone networks and the Internet.
Definition of the second
The second, symbol ''s'', is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be when expressed in the unit Hz, which is equal to s−1.Electric power and electronics
Caesium vapour thermionic generators are low-power devices that convert heat energy to electrical energy. In the two-electrode vacuum tube converter, caesium neutralizes the space charge near the cathode and enhances the current flow. Caesium is also important for its photoemissive properties, converting light to electron flow. It is used inphotoelectric cells
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
because caesium-based cathodes, such as the intermetallic compound , have a low threshold voltage for emission of electrons. The range of photoemissive devices using caesium include optical character recognition devices, photomultiplier tubes
Photomultiplier tubes (photomultipliers or PMTs for short) are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum. They are members of the class of vacuum tubes, more speci ...
, and video camera tubes. Nevertheless, germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, rubidium, selenium, silicon, tellurium, and several other elements can be substituted for caesium in photosensitive materials.
Caesium iodide (CsI), bromide (CsBr) and caesium fluoride (CsF) crystals are employed for scintillator
A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed ...
s in scintillation counter
A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.
It consists of a scintillator w ...
s widely used in mineral exploration and particle physics research to detect gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter re ...
and X-ray radiation. Being a heavy element, caesium provides good stopping power with better detection. Caesium compounds may provide a faster response (CsF) and be less hygroscopic (CsI).
Caesium vapour is used in many common magnetometer
A magnetometer is a device that measures magnetic field or magnetic dipole moment. Different types of magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location. A compass is one such device, o ...
s.
The element is used as an internal standard in spectrophotometry. Like other alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s, caesium has a great affinity for oxygen and is used as a " getter" in vacuum tubes. Other uses of the metal include high-energy lasers, vapour glow lamps, and vapour rectifier
A rectifier is an electrical device that converts alternating current (AC), which periodically reverses direction, to direct current (DC), which flows in only one direction. The reverse operation (converting DC to AC) is performed by an Power ...
s.
Centrifugation fluids
The high density of the caesium ion makes solutions of caesium chloride, caesium sulfate, and caesiumtrifluoroacetate
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a ...
() useful in molecular biology for density gradient ultracentrifugation. This technology is used primarily in the isolation of viral particles, subcellular organelle
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as organs are to the body, hence ''organelle,'' the ...
s and fractions, and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s from biological samples.
Chemical and medical use
 Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as
Relatively few chemical applications use caesium. Doping with caesium compounds enhances the effectiveness of several metal-ion catalysts for chemical synthesis, such as acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
, anthraquinone, ethylene oxide, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
, phthalic anhydride, styrene, methyl methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA ...
monomers, and various olefins
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. It is also used in the catalytic conversion of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
into sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
in the production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
.
Caesium fluoride
Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electroposit ...
enjoys a niche use in organic chemistry as a base and as an anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
source of fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
ion. Caesium salts sometimes replace potassium or sodium salts in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, such as cyclization, esterification, and polymerization. Caesium has also been used in thermoluminescent radiation dosimetry (TLD): When exposed to radiation, it acquires crystal defects that, when heated, revert with emission of light proportionate to the received dose. Thus, measuring the light pulse with a photomultiplier tube
Photomultiplier tubes (photomultipliers or PMTs for short) are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum. They are members of the class of vacuum tubes, more specif ...
can allow the accumulated radiation dose to be quantified.
Nuclear and isotope applications
Caesium-137 is a radioisotope commonly used as agamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter re ...
-emitter in industrial applications. Its advantages include a half-life of roughly 30 years, its availability from the nuclear fuel cycle, and having 137Ba as a stable end product. The high water solubility is a disadvantage which makes it incompatible with large pool irradiators for food and medical supplies. It has been used in agriculture, cancer treatment, and the sterilization of food, sewage sludge, and surgical equipment. Radioactive isotopes of caesium in radiation devices were used in the medical field to treat certain types of cancer, but emergence of better alternatives and the use of water-soluble caesium chloride in the sources, which could create wide-ranging contamination, gradually put some of these caesium sources out of use. Caesium-137 has been employed in a variety of industrial measurement gauges, including moisture, density, levelling, and thickness gauges. It has also been used in well logging devices for measuring the electron density of the rock formations, which is analogous to the bulk density of the formations.
Caesium-137 has been used in hydrologic studies analogous to those with tritium. As a daughter product of fission bomb testing from the 1950s through the mid-1980s, caesium-137 was released into the atmosphere, where it was absorbed readily into solution. Known year-to-year variation within that period allows correlation with soil and sediment layers. Caesium-134, and to a lesser extent caesium-135, have also been used in hydrology to measure the caesium output by the nuclear power industry. While they are less prevalent than either caesium-133 or caesium-137, these bellwether isotopes are produced solely from anthropogenic sources.
Other uses
ion engines
An ion thruster, ion drive, or ion engine is a form of electric propulsion used for spacecraft propulsion. It creates thrust by accelerating ions using electricity.
An ion thruster ionizes a neutral gas by extracting some electrons out of ...
designed for spacecraft propulsion
Spacecraft propulsion is any method used to accelerate spacecraft and artificial satellites. In-space propulsion exclusively deals with propulsion systems used in the vacuum of space and should not be confused with space launch or atmospheric e ...
on very long interplanetary or extraplanetary missions. The fuel was ionized by contact with a charged tungsten electrode. But corrosion by caesium on spacecraft components has pushed development in the direction of inert gas propellants, such as xenon, which are easier to handle in ground-based tests and do less potential damage to the spacecraft. Xenon was used in the experimental spacecraft Deep Space 1 launched in 1998. Nevertheless, field-emission electric propulsion thrusters that accelerate liquid metal ions such as caesium have been built.
Caesium nitrate
Caesium nitrate or cesium nitrate is a salt with the chemical formula Cs N O3. An alkali metal nitrate, it is used in pyrotechnic compositions, as a colorant and an oxidizer, e.g. in decoys and illumination flares. The caesium emissions are chi ...
is used as an oxidizer and pyrotechnic colorant to burn silicon in infrared flares
A flare, also sometimes called a fusée, fusee, or bengala in some Latin-speaking countries, is a type of pyrotechnic that produces a bright light or intense heat without an explosion. Flares are used for distress signaling, illumination, o ...
, such as the LUU-19 flare, because it emits much of its light in the near infrared spectrum. Caesium compounds may have been used as fuel additives to reduce the radar signature of exhaust plumes in the Lockheed A-12 CIA
The Central Intelligence Agency (CIA ), known informally as the Agency and historically as the Company, is a civilian intelligence agency, foreign intelligence service of the federal government of the United States, officially tasked with gat ...
reconnaissance aircraft. Caesium and rubidium have been added as a carbonate to glass because they reduce electrical conductivity and improve stability and durability of fibre optics and night vision
Night vision is the ability to see in low-light conditions, either naturally with scotopic vision or through a night-vision device. Night vision requires both sufficient spectral range and sufficient intensity range. Humans have poor night vi ...
devices. Caesium fluoride or caesium aluminium fluoride are used in fluxes formulated for brazing aluminium alloys that contain magnesium.
Magnetohydrodynamic (MHD) power-generating systems were researched, but failed to gain widespread acceptance. Caesium metal has also been considered as the working fluid in high-temperature Rankine cycle turboelectric generators.
Caesium salts have been evaluated as antishock reagents following the administration of arsenical drugs. Because of their effect on heart rhythms, however, they are less likely to be used than potassium or rubidium salts. They have also been used to treat epilepsy.
Caesium-133 can be laser cooled and used to probe fundamental and technological problems in quantum physics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
. It has a particularly convenient Feshbach Feshbach is a surname. Notable people with the surname include:
* Herman Feshbach (1917–2000), American physicist
* Jessica Feshbach (born 1975), American Scientologist
* Murray Feshbach (1929–2019), American demographer
See also
* Fischbach ( ...
spectrum to enable studies of ultracold atoms requiring tunable interactions.
Health and safety hazards
arrhythmia
Arrhythmias, also known as cardiac arrhythmias, heart arrhythmias, or dysrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults ...
, and acute cardiac arrest, but such amounts would not ordinarily be encountered in natural sources.
The median lethal dose
In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a toxin, radiation, or pathogen. The value of LD50 for a substance is the ...
(LD50) for caesium chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each ...
in mice is 2.3 g per kilogram, which is comparable to the LD50 values of potassium chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt ...
and sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. The principal use of nonradioactive caesium is as caesium formate in petroleum drilling fluids because it is much less toxic than alternatives, though it is more costly.
Caesium metal is one of the most reactive elements and is highly explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An expl ...
in the presence of water. The hydrogen gas produced by the reaction is heated by the thermal energy released at the same time, causing ignition and a violent explosion. This can occur with other alkali metals, but caesium is so potent that this explosive reaction can be triggered even by cold water.
It is highly pyrophoric: the autoignition temperature
The autoignition temperature or kindling point of a substance is the lowest temperature in which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to su ...
of caesium is , and it ignites explosively in air to form caesium hydroxide and various oxides. Caesium hydroxide is a very strong base, and will rapidly corrode glass.
The isotopes 134 134 may refer to:
* 134 (number)
* AD 134
* 134 BC
* 134 (MBTA bus)
*134 (New Jersey bus) 134 may refer to:
*134 (number)
* AD 134
*134 BC
*134 (MBTA bus)
The Massachusetts Bay Transportation Authority bus division operates bus routes in the B ...
and 137 are present in the biosphere in small amounts from human activities, differing by location. Radiocaesium does not accumulate in the body as readily as other fission products (such as radioiodine and radiostrontium). About 10% of absorbed radiocaesium washes out of the body relatively quickly in sweat and urine. The remaining 90% has a biological half-life between 50 and 150 days. Radiocaesium follows potassium and tends to accumulate in plant tissues, including fruits and vegetables. Plants vary widely in the absorption of caesium, sometimes displaying great resistance to it. It is also well-documented that mushrooms from contaminated forests accumulate radiocaesium (caesium-137) in the fungal sporocarps. Accumulation of caesium-137 in lakes has been a great concern after the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
. Experiments with dogs showed that a single dose of 3.8 millicuries (140 MBq, 4.1 μg of caesium-137) per kilogram is lethal within three weeks; smaller amounts may cause infertility and cancer. The International Atomic Energy Agency
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 1957 ...
and other sources have warned that radioactive materials, such as caesium-137, could be used in radiological dispersion devices, or " dirty bombs".
See also
* * Acerinox accident, a caesium-137 contamination accident in 1998 * Goiânia accident, a major radioactive contamination incident in 1987 involving caesium-137 *Kramatorsk radiological accident
The Kramatorsk radiological accident was a radiation accident that happened in Kramatorsk, in the Ukrainian SSR from 1980 to 1989. A small capsule containing highly radioactive caesium-137 was found inside the concrete wall of an apartment build ...
, a 137Cs lost-source incident between 1980 and 1989
Notes
References
External links
Caesium or Cesium
at '' The Periodic Table of Videos'' (University of Nottingham)
View the reaction of Caesium (most reactive metal in the periodic table) with Fluorine (most reactive non-metal)
courtesy of The Royal Institution. * {{Authority control Alkali metals Chemical elements with body-centered cubic structure Chemical elements Glycine receptor agonists Reducing agents Articles containing video clips