|
Atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules. The atmosphere of Earth is composed of nitrogen (78%), oxygen (21%), argon (0.9%), carbon dioxide (0.04%) and trace gases. Most organisms use oxygen for respiration; lightning and bacteria perform nitrogen fixation which produces ammonia that is used to make nucleotides and amino acids; plants, algae, and cyanobacteria use carbon dioxide for photosynthesis. The layered composition of the atmosphere minimises the harmful effects of sunlight, ultraviolet radiation, solar wind, and cosmic rays and thus protects the organisms from genetic damage. The curr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Atmosphere Of Earth
The atmosphere of Earth is composed of a layer of gas mixture that surrounds the Earth's planetary surface (both lands and oceans), known collectively as air, with variable quantities of suspended aerosols and particulates (which create weather features such as clouds and hazes), all retained by gravity of Earth, Earth's gravity. The atmosphere serves as a protective buffer between the Earth's surface and outer space, shields the surface from most meteoroids and ultraviolet solar irradiance, solar radiation, keeps it warm and reduces diurnal temperature variation (temperature extremes between daytime, day and night) through heat retention (greenhouse effect), redistributes heat and moisture among different regions via air currents, and provides the atmospheric chemistry, chemical and climate conditions allowing life to exist and evolution, evolve on Earth. By mole fraction (i.e., by quantity of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Dioxide In Earth's Atmosphere
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of three main greenhouse gases in the atmosphere of Earth. The concentration of carbon dioxide () in the atmosphere reached 427 ppm (0.0427%) on a molar basis in 2024, representing 3341 gigatonnes of . This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century. The increase is due to human activity. The current increase in concentrations is primarily driven by the burning of fossil fuels.IPCC (2022Summary for policy makers iClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Other significant human activities that emit include cem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cosmic Ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own galaxy, and from distant galaxies. Upon impact with Earth's atmosphere, cosmic rays produce showers of secondary particles, some of which reach the surface, although the bulk are deflected off into space by the magnetosphere or the heliosphere. Cosmic rays were discovered by Victor Hess in 1912 in balloon experiments, for which he was awarded the 1936 Nobel Prize in Physics. Direct measurement of cosmic rays, especially at lower energies, has been possible since the launch of the first satellites in the late 1950s. Particle detectors similar to those used in nuclear and high-energy physics are used on satellites and space probes for research into cosmic rays. Data from the Fermi Space Telescope (2013) have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrared (typically perceived by humans as warmth) and ultraviolet (which can have physiological effects such as sunburn) lights. However, according to the American Meteorological Society, there are "conflicting conventions as to whether all three [...] are referred to as light, or whether that term should only be applied to the visible portion of the spectrum." Upon reaching the Earth, sunlight is light scattering by particles, scattered and attenuation, filtered through the atmosphere of Earth, Earth's atmosphere as daylight when the Sun is above the horizon. When direct solar radiation is not blocked by clouds, it is experienced as sunshine, a combination of bright light and radiant heat (atmospheric). When cloud cover, blocked by clouds or dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Paleoatmosphere
A paleoatmosphere (or ''palaeoatmosphere'') is an atmosphere, particularly that of Earth, at some unspecified time in the geological past. When regarding geological history of Earth, the paleoatmosphere can be chronologically divided into the Hadean ''first atmosphere'', which resembled the compositions of the solar nebula; the Archean ''second atmosphere'' (also known as the prebiotic atmosphere), which became nitrogen-abundant due to volcanic outgassing and meteoric injections during the Late Heavy Bombardment; and the Proterozoic and Phanerozoic ''third atmosphere'', which started to contain free oxygen due to biotic photosynthesis. Composition The composition of Earth's paleoatmosphere can be inferred today from the study of the abundance of proxy materials such as iron oxides and charcoal and the fossil data, such as the stomatal density of fossil leaves in geological deposits. Although today's atmosphere is dominated by nitrogen (about 78%), oxygen (abo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
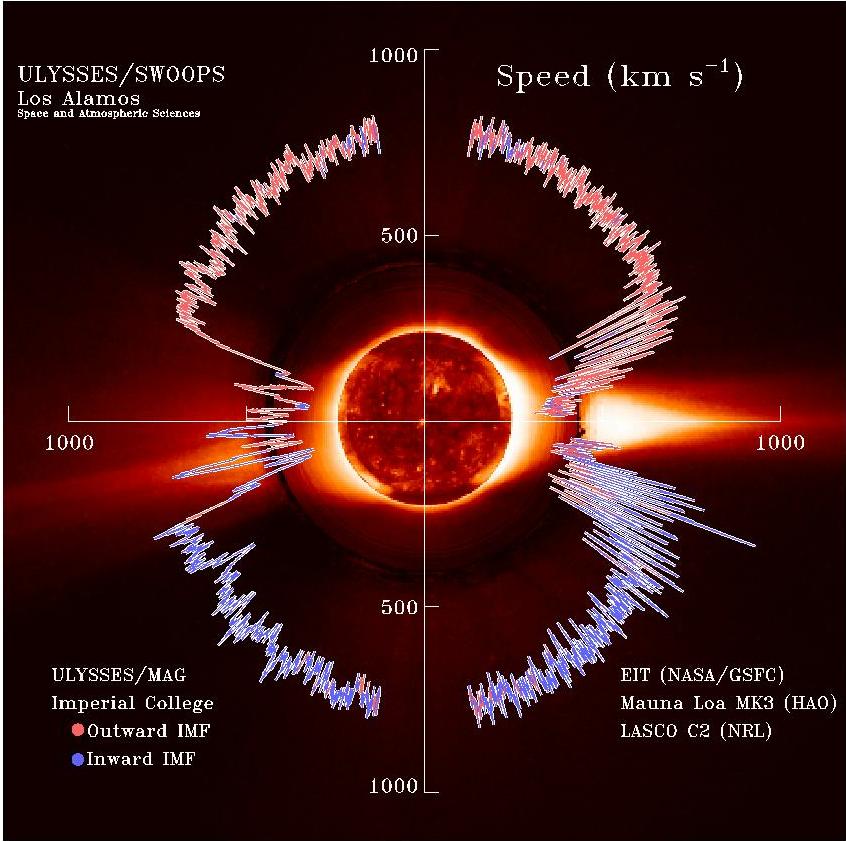
Solar Wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of particle species found in the solar plasma: trace amounts of heavy ions and atomic nuclei of Chemical element, elements such as carbon, nitrogen, oxygen, neon, magnesium, silicon, sulfur, and iron. There are also rarer traces of some other nuclei and isotopes such as phosphorus, titanium, chromium, and nickel's isotopes 58Ni, 60Ni, and 62Ni. Superimposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over Solar coordinate systems#Heliographic, solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. ''Photosynthesis'' usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth. Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cyanobacteria
Cyanobacteria ( ) are a group of autotrophic gram-negative bacteria that can obtain biological energy via oxygenic photosynthesis. The name "cyanobacteria" () refers to their bluish green (cyan) color, which forms the basis of cyanobacteria's informal common name, blue-green algae. Cyanobacteria are probably the most numerous taxon to have ever existed on Earth and the first organisms known to have produced oxygen, having appeared in the middle Archean eon and apparently originated in a freshwater or terrestrial environment. Their photopigments can absorb the red- and blue-spectrum frequencies of sunlight (thus reflecting a greenish color) to split water molecules into hydrogen ions and oxygen. The hydrogen ions are used to react with carbon dioxide to produce complex organic compounds such as carbohydrates (a process known as carbon fixation), and the oxygen is released as a byproduct. By continuously producing and releasing oxygen over billions of years, cyanobacte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |