chemistry on:
[Wikipedia]
[Google]
[Amazon]
 Chemistry is the
Chemistry is the
 The current model of atomic structure is the
The current model of atomic structure is the  A
A
 The
The
 A chemical element is a pure substance which is composed of a single type of atom, characterized by its particular number of
A chemical element is a pure substance which is composed of a single type of atom, characterized by its particular number of
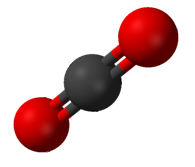 A ''compound'' is a pure chemical substance composed of more than one element. The properties of a compound bear little similarity to those of its elements. The standard nomenclature of compounds is set by the
A ''compound'' is a pure chemical substance composed of more than one element. The properties of a compound bear little similarity to those of its elements. The standard nomenclature of compounds is set by the
 A ''molecule'' is the smallest indivisible portion of a pure
A ''molecule'' is the smallest indivisible portion of a pure  The "inert" or noble gas elements (
The "inert" or noble gas elements (
 In addition to the specific chemical properties that distinguish different chemical classifications, chemicals can exist in several phases. For the most part, the chemical classifications are independent of these bulk phase classifications; however, some more exotic phases are incompatible with certain chemical properties. A ''phase'' is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as
In addition to the specific chemical properties that distinguish different chemical classifications, chemicals can exist in several phases. For the most part, the chemical classifications are independent of these bulk phase classifications; however, some more exotic phases are incompatible with certain chemical properties. A ''phase'' is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as
 Atoms sticking together in molecules or crystals are said to be bonded with one another. A chemical bond may be visualized as the
Atoms sticking together in molecules or crystals are said to be bonded with one another. A chemical bond may be visualized as the  In a covalent bond, one or more pairs of
In a covalent bond, one or more pairs of
 The term
The term
 When a chemical substance is transformed as a result of its interaction with another substance or with energy, a chemical reaction is said to have occurred. A ''chemical reaction'' is therefore a concept related to the "reaction" of a substance when it comes in close contact with another, whether as a mixture or a
When a chemical substance is transformed as a result of its interaction with another substance or with energy, a chemical reaction is said to have occurred. A ''chemical reaction'' is therefore a concept related to the "reaction" of a substance when it comes in close contact with another, whether as a mixture or a
 An ''ion'' is a charged species, an atom or a molecule, that has lost or gained one or more electrons. When an atom loses an electron and thus has more protons than electrons, the atom is a positively charged ion or
An ''ion'' is a charged species, an atom or a molecule, that has lost or gained one or more electrons. When an atom loses an electron and thus has more protons than electrons, the atom is a positively charged ion or
 A substance can often be classified as an acid or a base. There are several different theories which explain acid–base behavior. The simplest is
A substance can often be classified as an acid or a base. There are several different theories which explain acid–base behavior. The simplest is
 Early civilizations, such as the
Early civilizations, such as the  In the
In the  In the following decades, many important discoveries were made, such as the nature of 'air' which was discovered to be composed of many different gases. The Scottish chemist
In the following decades, many important discoveries were made, such as the nature of 'air' which was discovered to be composed of many different gases. The Scottish chemist  At the turn of the twentieth century the theoretical underpinnings of chemistry were finally understood due to a series of remarkable discoveries that succeeded in probing and discovering the very nature of the internal structure of atoms. In 1897,
At the turn of the twentieth century the theoretical underpinnings of chemistry were finally understood due to a series of remarkable discoveries that succeeded in probing and discovering the very nature of the internal structure of atoms. In 1897,
General Chemistry principles, patterns and applications
{{Authority control Chemistry,
 Chemistry is the
Chemistry is the scientific
Science is a systematic endeavor that builds and organizes knowledge in the form of testable explanations and predictions about the universe.
Science may be as old as the human species, and some of the earliest archeological evidence for ...
study of the properties and behavior of matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic partic ...
. It is a natural science
Natural science is one of the branches of science concerned with the description, understanding and prediction of natural phenomena, based on empirical evidence from observation and experimentation. Mechanisms such as peer review and repeatab ...
that covers the elements that make up matter to the compounds made of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s, molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s and ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s: their composition, structure, properties, behavior and the changes they undergo during a reaction
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and me ...
with other substances. Chemistry also addresses the nature of chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s in chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s.
In the scope of its subject, chemistry occupies an intermediate position between physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
and biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
. It is sometimes called the central science
Chemistry is often called the central science because of its role in connecting the physical sciences, which include chemistry, with the life sciences and applied sciences such as medicine and engineering. The nature of this relationship is one o ...
because it provides a foundation for understanding both basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany
Botany, also called , plant biology or phytology, is the science of plant life and a branch of biology. A botanist, plant scientist or phytologist is a scientist who specialises in this field. The term "botany" comes from the Ancient Greek w ...
), the formation of igneous rocks (geology
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Ear ...
), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology
Ecology () is the study of the relationships between living organisms, including humans, and their physical environment. Ecology considers organisms at the individual, population, community, ecosystem, and biosphere level. Ecology overlaps wi ...
), the properties of the soil on the moon (cosmochemistry
Cosmochemistry (from Greek κόσμος ''kósmos'', "universe" and χημεία ''khemeía'') or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is done ...
), how medications work (pharmacology
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemica ...
), and how to collect DNA evidence at a crime scene (forensic
Forensic science, also known as criminalistics, is the application of science to Criminal law, criminal and Civil law (legal system), civil laws, mainly—on the criminal side—during criminal investigation, as governed by the legal standard ...
s).
Etymology
The word ''chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
'' comes from a modification during the Renaissance of the word ''alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
,'' which referred to an earlier set of practices that encompassed elements of chemistry, metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
, philosophy
Philosophy (from , ) is the systematized study of general and fundamental questions, such as those about existence, reason, knowledge, values, mind, and language. Such questions are often posed as problems to be studied or resolved. Some ...
, astrology
Astrology is a range of Divination, divinatory practices, recognized as pseudoscientific since the 18th century, that claim to discern information about human affairs and terrestrial events by studying the apparent positions of Celestial o ...
, astronomy
Astronomy () is a natural science that studies astronomical object, celestial objects and phenomena. It uses mathematics, physics, and chemistry in order to explain their origin and chronology of the Universe, evolution. Objects of interest ...
, mysticism
Mysticism is popularly known as becoming one with God or the Absolute, but may refer to any kind of ecstasy or altered state of consciousness which is given a religious or spiritual meaning. It may also refer to the attainment of insight in u ...
and medicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pract ...
. Alchemy is often associated with the quest to turn lead or other base metals into gold, though alchemists were also interested in many of the questions of modern chemistry.
The modern word ''alchemy'' in turn is derived from the Arabic
Arabic (, ' ; , ' or ) is a Semitic languages, Semitic language spoken primarily across the Arab world.Semitic languages: an international handbook / edited by Stefan Weninger; in collaboration with Geoffrey Khan, Michael P. Streck, Janet C ...
word (). This may have Egyptian
Egyptian describes something of, from, or related to Egypt.
Egyptian or Egyptians may refer to:
Nations and ethnic groups
* Egyptians, a national group in North Africa
** Egyptian culture, a complex and stable culture with thousands of years of ...
origins since is derived from the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic peri ...
, which is in turn derived from the word , which is the ancient name of Egypt in the Egyptian language."alchemy", entry in ''The Oxford English Dictionary'', J.A. Simpson and E.S.C. Weiner, vol. 1, 2nd ed., 1989, . Alternately, may derive from 'cast together'.
Modern principles
 The current model of atomic structure is the
The current model of atomic structure is the quantum mechanical model
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
. Traditional chemistry starts with the study of elementary particles
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. Particles currently thought to be elementary include electrons, the fundamental fermions (quarks, leptons, anti ...
, atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s, molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s, substances, metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s, crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s and other aggregates of matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic partic ...
. Matter can be studied in solid, liquid, gas and plasma states, in isolation or in combination. The interactions
Interaction is action that occurs between two or more objects, with broad use in philosophy and the sciences. It may refer to:
Science
* Interaction hypothesis, a theory of second language acquisition
* Interaction (statistics)
* Interactions o ...
, reactions
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and me ...
and transformations that are studied in chemistry are usually the result of interactions between atoms, leading to rearrangements of the chemical bonds which hold atoms together. Such behaviors are studied in a chemistry laboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratory services are provided in a variety of settings: physicia ...
.
The chemistry laboratory stereotypically uses various forms of laboratory glassware
Laboratory glassware refers to a variety of equipment used in scientific work, and traditionally made of glass. Glass can be blown, bent, cut, molded, and formed into many sizes and shapes, and is therefore common in chemistry, biology, and anal ...
. However glassware is not central to chemistry, and a great deal of experimental (as well as applied/industrial) chemistry is done without it.
 A
A chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
is a transformation of some substances into one or more different substances. The basis of such a chemical transformation is the rearrangement of electrons in the chemical bonds between atoms. It can be symbolically depicted through a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
, which usually involves atoms as subjects. The number of atoms on the left and the right in the equation for a chemical transformation is equal. (When the number of atoms on either side is unequal, the transformation is referred to as a nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
or radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
.) The type of chemical reactions a substance may undergo and the energy changes that may accompany it are constrained by certain basic rules, known as chemical law
Chemical laws are those laws of nature relevant to chemistry. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical re ...
s.
Energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
considerations are invariably important in almost all chemical studies. Chemical substances are classified in terms of their structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
, phase, as well as their chemical composition
A chemical composition specifies the identity, arrangement, and ratio of the elements making up a compound.
Chemical formulas can be used to describe the relative amounts of elements present in a compound. For example, the chemical formula for ...
s. They can be analyzed using the tools of chemical analysis
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separati ...
, e.g. spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
and chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
. Scientists engaged in chemical research are known as chemists
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
. Most chemists specialize in one or more sub-disciplines. Several concept
Concepts are defined as abstract ideas. They are understood to be the fundamental building blocks of the concept behind principles, thoughts and beliefs.
They play an important role in all aspects of cognition. As such, concepts are studied by s ...
s are essential for the study of chemistry; some of them are:
Matter
In chemistry, matter is defined as anything that hasrest mass
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, i ...
and volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). The de ...
(it takes up space) and is made up of particle
In the Outline of physical science, physical sciences, a particle (or corpuscule in older texts) is a small wikt:local, localized physical body, object which can be described by several physical property, physical or chemical property, chemical ...
s. The particles that make up matter have rest mass as well – not all particles have rest mass, such as the photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they always ...
. Matter can be a pure chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
or a mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
of substances.
Atom
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
is the basic unit of chemistry. It consists of a dense core called the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron i ...
surrounded by a space occupied by an electron cloud
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
. The nucleus is made up of positively charged protons
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
and uncharged neutrons
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave ...
(together called nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons were ...
s), while the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
cloud consists of negatively charged electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s which orbit the nucleus. In a neutral atom, the negatively charged electrons balance out the positive charge of the protons. The nucleus is dense; the mass of a nucleon is approximately 1,836 times that of an electron, yet the radius of an atom is about 10,000 times that of its nucleus.
The atom is also the smallest entity that can be envisaged to retain the chemical properties
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley, ...
of the element, such as electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
, ionization potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
, preferred oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
(s), coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central i ...
, and preferred types of bonds to form (e.g., metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
lic, ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
ic, covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
).
Element
proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s in the nuclei of its atoms, known as the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
and represented by the symbol ''Z''. The mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
is the sum of the number of protons and neutrons in a nucleus. Although all the nuclei of all atoms belonging to one element will have the same atomic number, they may not necessarily have the same mass number; atoms of an element which have different mass numbers are known as isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
s. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
, but atoms of carbon may have mass numbers of 12 or 13.
The standard presentation of the chemical elements is in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, which orders elements by atomic number. The periodic table is arranged in groups
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic ide ...
, or columns, and periods, or rows. The periodic table is useful in identifying periodic trends
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
.
Compound
 A ''compound'' is a pure chemical substance composed of more than one element. The properties of a compound bear little similarity to those of its elements. The standard nomenclature of compounds is set by the
A ''compound'' is a pure chemical substance composed of more than one element. The properties of a compound bear little similarity to those of its elements. The standard nomenclature of compounds is set by the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC). Organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s are named according to the organic nomenclature
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of ...
system. The names for inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
s are created according to the inorganic nomenclature
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in '' Nomencla ...
system. When a compound has more than one component, then they are divided into two classes, the electropositive and the electronegative components. In addition the Chemical Abstracts Service
CAS (formerly Chemical Abstracts Service) is a division of the American Chemical Society. It is a source of chemical information. CAS is located in Columbus, Ohio, United States.
Print periodicals
''Chemical Abstracts'' is a periodical index tha ...
has devised a method to index chemical substances. In this scheme each chemical substance is identifiable by a number known as its CAS registry number
A CAS Registry Number (also referred to as CAS RN or informally CAS Number) is a unique identification number assigned by the Chemical Abstracts Service (CAS), US to every chemical substance described in the open scientific literature. It inclu ...
.
Molecule
 A ''molecule'' is the smallest indivisible portion of a pure
A ''molecule'' is the smallest indivisible portion of a pure chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
that has its unique set of chemical properties, that is, its potential to undergo a certain set of chemical reactions with other substances. However, this definition only works well for substances that are composed of molecules, which is not true of many substances (see below). Molecules are typically a set of atoms bound together by covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s, such that the structure is electrically neutral and all valence electrons are paired with other electrons either in bonds or in lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s.
Thus, molecules exist as electrically neutral units, unlike ions. When this rule is broken, giving the "molecule" a charge, the result is sometimes named a molecular ion
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It i ...
or a polyatomic ion. However, the discrete and separate nature of the molecular concept usually requires that molecular ions be present only in well-separated form, such as a directed beam in a vacuum in a mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
. Charged polyatomic collections residing in solids (for example, common sulfate or nitrate ions) are generally not considered "molecules" in chemistry. Some molecules contain one or more unpaired electrons, creating radicals. Most radicals are comparatively reactive, but some, such as nitric oxide (NO) can be stable.
helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
, argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
, krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often ...
, xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through ...
) are composed of lone atoms as their smallest discrete unit, but the other isolated chemical elements consist of either molecules or networks of atoms bonded to each other in some way. Identifiable molecules compose familiar substances such as water, air, and many organic compounds like alcohol, sugar, gasoline, and the various pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and re ...
s.
However, not all substances or chemical compounds consist of discrete molecules, and indeed most of the solid substances that make up the solid crust, mantle, and core of the Earth are chemical compounds without molecules. These other types of substances, such as ionic compounds
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged io ...
and network solids A network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) is a chemical compound (or element) in which the atoms are bonded by covalent bonds in a continuous network extending throughout the ma ...
, are organized in such a way as to lack the existence of identifiable molecules ''per se''. Instead, these substances are discussed in terms of formula unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. E ...
s or unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
s as the smallest repeating structure within the substance. Examples of such substances are mineral salts (such as table salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
), solids like carbon and diamond, metals, and familiar silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
and silicate minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, silica (silicon dioxide, ) is usually con ...
such as quartz and granite.
One of the main characteristics of a molecule is its geometry often called its structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. While the structure of diatomic, triatomic or tetra-atomic molecules may be trivial, (linear, angular pyramidal etc.) the structure of polyatomic molecules, that are constituted of more than six atoms (of several elements) can be crucial for its chemical nature.
Substance and mixture
A chemical substance is a kind of matter with a definitecomposition
Composition or Compositions may refer to:
Arts and literature
*Composition (dance), practice and teaching of choreography
*Composition (language), in literature and rhetoric, producing a work in spoken tradition and written discourse, to include v ...
and set of properties
Property is the ownership of land, resources, improvements or other tangible objects, or intellectual property.
Property may also refer to:
Mathematics
* Property (mathematics)
Philosophy and science
* Property (philosophy), in philosophy and ...
. A collection of substances is called a mixture. Examples of mixtures are air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
and alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
s.
Mole and amount of substance
The mole is a unit of measurement that denotes anamount of substance
In chemistry, the amount of substance ''n'' in a given sample of matter is defined as the quantity or number of discrete atomic-scale particles in it divided by the Avogadro constant ''N''A. The particles or entities may be molecules, atoms, ions, ...
(also called chemical amount). One mole is defined to contain exactly particles (atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s, molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s, ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s, or electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s), where the number of particles
The particle number (or number of particles) of a thermodynamic system, conventionally indicated with the letter ''N'', is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is ...
per mole is known as the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining con ...
. Molar concentration
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of solut ...
is the amount of a particular substance per volume of solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
, and is commonly reported in mol/ dm3.
Phase
pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
or temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
.
Physical properties, such as density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
and refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, or ...
tend to fall within values characteristic of the phase. The phase of matter is defined by the ''phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
'', which is when energy put into or taken out of the system goes into rearranging the structure of the system, instead of changing the bulk conditions.
Sometimes the distinction between phases can be continuous instead of having a discrete boundary' in this case the matter is considered to be in a supercritical state. When three states meet based on the conditions, it is known as a triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the subli ...
and since this is invariant, it is a convenient way to define a set of conditions.
The most familiar examples of phases are solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
s, liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
s, and gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
es. Many substances exhibit multiple solid phases. For example, there are three phases of solid iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
(alpha, gamma, and delta) that vary based on temperature and pressure. A principal difference between solid phases is the crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
, or arrangement, of the atoms. Another phase commonly encountered in the study of chemistry is the ''aqueous'' phase, which is the state of substances dissolved in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
(that is, in water).
Less familiar phases include plasmas, Bose–Einstein condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.67&n ...
s and fermionic condensate
A fermionic condensate or Fermi–Dirac condensate is a superfluid phase formed by fermionic particles at low temperatures. It is closely related to the Bose–Einstein condensate, a superfluid phase formed by bosonic atoms under similar condit ...
s and the paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, d ...
and ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials ...
phases of magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, ...
ic materials. While most familiar phases deal with three-dimensional systems, it is also possible to define analogs in two-dimensional systems, which has received attention for its relevance to systems in biology
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary i ...
.
Bonding
 Atoms sticking together in molecules or crystals are said to be bonded with one another. A chemical bond may be visualized as the
Atoms sticking together in molecules or crystals are said to be bonded with one another. A chemical bond may be visualized as the multipole
A multipole expansion is a Series (mathematics), mathematical series representing a Function (mathematics), function that depends on angles—usually the two angles used in the spherical coordinate system (the polar and Azimuth, azimuthal angles) f ...
balance between the positive charges in the nuclei and the negative charges oscillating about them. More than simple attraction and repulsion, the energies and distributions characterize the availability of an electron to bond to another atom.
The chemical bond can be a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
, an ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. ...
, a hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
or just because of Van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
. Each of these kinds of bonds is ascribed to some potential. These potentials create the interactions
Interaction is action that occurs between two or more objects, with broad use in philosophy and the sciences. It may refer to:
Science
* Interaction hypothesis, a theory of second language acquisition
* Interaction (statistics)
* Interactions o ...
which hold atoms together in molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s or crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s. In many simple compounds, valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
, the Valence Shell Electron Pair Repulsion model (VSEPR
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm the ...
), and the concept of oxidation number
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
can be used to explain molecular structure and composition.
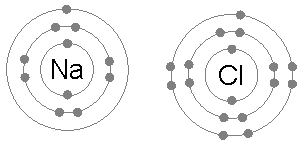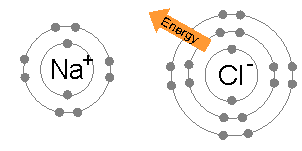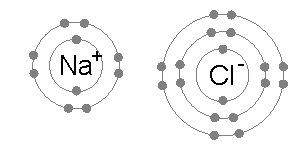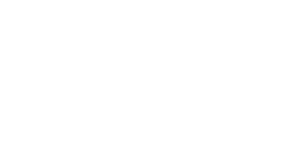
An ionic bond is formed when a metal loses one or more of its electrons, becoming a positively charged cation, and the electrons are then gained by the non-metal atom, becoming a negatively charged anion. The two oppositely charged ions attract one another, and the ionic bond is the electrostatic force of attraction between them. For example, sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
(Na), a metal, loses one electron to become an Na+ cation while chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
(Cl), a non-metal, gains this electron to become Cl−. The ions are held together due to electrostatic attraction, and that compound sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(NaCl), or common table salt, is formed.
valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s are shared by two atoms: the resulting electrically neutral group of bonded atoms is termed a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
. Atoms will share valence electrons in such a way as to create a noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
electron configuration (eight electrons in their outermost shell) for each atom. Atoms that tend to combine in such a way that they each have eight electrons in their valence shell are said to follow the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule i ...
. However, some elements like hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
need only two electrons in their outermost shell to attain this stable configuration; these atoms are said to follow the ''duet rule'', and in this way they are reaching the electron configuration of the noble gas helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, which has two electrons in its outer shell.
Similarly, theories from classical physics
Classical physics is a group of physics theories that predate modern, more complete, or more widely applicable theories. If a currently accepted theory is considered to be modern, and its introduction represented a major paradigm shift, then the ...
can be used to predict many ionic structures. With more complicated compounds, such as metal complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
, valence bond theory is less applicable and alternative approaches, such as the molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
theory, are generally used. See diagram on electronic orbitals.
Energy
In the context of chemistry, energy is an attribute of a substance as a consequence of its atomic,molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
or aggregate structure
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as ...
. Since a chemical transformation is accompanied by a change in one or more of these kinds of structures, it is invariably accompanied by an increase or decrease of energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
of the substances involved. Some energy is transferred between the surroundings and the reactants of the reaction in the form of heat or light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 tera ...
; thus the products of a reaction may have more or less energy than the reactants.
A reaction is said to be exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs fr ...
if the final state is lower on the energy scale than the initial state; in the case of endergonic reaction
In chemical thermodynamics, an endergonic reaction (; also called a heat absorbing nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and an additional driving fo ...
s the situation is the reverse. A reaction is said to be exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
if the reaction releases heat to the surroundings; in the case of endothermic reaction
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p ...
s, the reaction absorbs heat from the surroundings.
Chemical reactions are invariably not possible unless the reactants surmount an energy barrier known as the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
. The ''speed'' of a chemical reaction (at given temperature T) is related to the activation energy E, by the Boltzmann's population factor – that is the probability of a molecule to have energy greater than or equal to E at the given temperature T. This exponential dependence of a reaction rate on temperature is known as the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
.
The activation energy necessary for a chemical reaction to occur can be in the form of heat, light, electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
or mechanical force
In physics, a force is an influence that can change the motion of an object. A force can cause an object with mass to change its velocity (e.g. moving from a state of rest), i.e., to accelerate. Force can also be described intuitively as a p ...
in the form of ultrasound
Ultrasound is sound waves with frequency, frequencies higher than the upper audible limit of human hearing range, hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hea ...
.
A related concept free energy, which also incorporates entropy considerations, is a very useful means for predicting the feasibility of a reaction and determining the state of equilibrium of a chemical reaction, in chemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurement ...
. A reaction is feasible only if the total change in the Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
is negative, ; if it is equal to zero the chemical reaction is said to be at equilibrium.
There exist only limited possible states of energy for electrons, atoms and molecules. These are determined by the rules of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
, which require quantization of energy of a bound system. The atoms/molecules in a higher energy state are said to be excited. The molecules/atoms of substance in an excited energy state are often much more reactive; that is, more amenable to chemical reactions.
The phase of a substance is invariably determined by its energy and the energy of its surroundings. When the intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s of a substance are such that the energy of the surroundings is not sufficient to overcome them, it occurs in a more ordered phase like liquid or solid as is the case with water (H2O); a liquid at room temperature because its molecules are bound by hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
. Whereas hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S) is a gas at room temperature and standard pressure, as its molecules are bound by weaker dipole-dipole interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s.
The transfer of energy from one chemical substance to another depends on the ''size'' of energy quanta emitted from one substance. However, heat energy is often transferred more easily from almost any substance to another because the phonons
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanic ...
responsible for vibrational and rotational energy levels in a substance have much less energy than photons
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they alway ...
invoked for the electronic energy transfer. Thus, because vibrational and rotational energy levels are more closely spaced than electronic energy levels, heat is more easily transferred between substances relative to light or other forms of electronic energy. For example, ultraviolet electromagnetic radiation is not transferred with as much efficacy from one substance to another as thermal or electrical energy.
The existence of characteristic energy levels for different chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
s is useful for their identification by the analysis of spectral lines
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to iden ...
. Different kinds of spectra are often used in chemical spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
, e.g. IR, microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ran ...
, NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
, ESR, etc. Spectroscopy is also used to identify the composition of remote objects – like stars and distant galaxies – by analyzing their radiation spectra.
chemical energy
Chemical energy is the energy of chemical substances that is released when they undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How ...
is often used to indicate the potential of a chemical substance to undergo a transformation through a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
or to transform other chemical substances.
Reaction
 When a chemical substance is transformed as a result of its interaction with another substance or with energy, a chemical reaction is said to have occurred. A ''chemical reaction'' is therefore a concept related to the "reaction" of a substance when it comes in close contact with another, whether as a mixture or a
When a chemical substance is transformed as a result of its interaction with another substance or with energy, a chemical reaction is said to have occurred. A ''chemical reaction'' is therefore a concept related to the "reaction" of a substance when it comes in close contact with another, whether as a mixture or a solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
; exposure to some form of energy, or both. It results in some energy exchange between the constituents of the reaction as well as with the system environment, which may be designed vessels—often laboratory glassware
Laboratory glassware refers to a variety of equipment used in scientific work, and traditionally made of glass. Glass can be blown, bent, cut, molded, and formed into many sizes and shapes, and is therefore common in chemistry, biology, and anal ...
.
Chemical reactions can result in the formation or dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
of molecules, that is, molecules breaking apart to form two or more molecules or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds. Oxidation, reduction, dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
, acid–base neutralization and molecular rearrangement are some examples of common chemical reactions.
A chemical reaction can be symbolically depicted through a chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
. While in a non-nuclear chemical reaction the number and kind of atoms on both sides of the equation are equal, for a nuclear reaction this holds true only for the nuclear particles viz. protons and neutrons.
The sequence of steps in which the reorganization of chemical bonds may be taking place in the course of a chemical reaction is called its mechanism
Mechanism may refer to:
* Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that ...
. A chemical reaction can be envisioned to take place in a number of steps, each of which may have a different speed. Many reaction intermediates
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary s ...
with variable stability can thus be envisaged during the course of a reaction. Reaction mechanisms are proposed to explain the kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
and the relative product mix of a reaction. Many physical chemists
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistical me ...
specialize in exploring and proposing the mechanisms of various chemical reactions. Several empirical rules, like the Woodward–Hoffmann rules The Woodward–Hoffmann rules (or the pericyclic selection rules), devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reac ...
often come in handy while proposing a mechanism for a chemical reaction.
According to the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
gold book, a chemical reaction is "a process that results in the interconversion of chemical species." Accordingly, a chemical reaction may be an elementary reaction
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state. In practice, a reaction is assumed to be elementary if no reaction in ...
or a stepwise reaction
In chemistry, a stepwise reaction (also called an overall reaction, complex reaction, and multistep reaction, among others) is a chemical reaction with one or more reaction intermediates, which by definition involves at least two consecutive elem ...
. An additional caveat is made, in that this definition includes cases where the interconversion of conformers is experimentally observable. Such detectable chemical reactions normally involve sets of molecular entities as indicated by this definition, but it is often conceptually convenient to use the term also for changes involving single molecular entities (i.e. 'microscopic chemical events').
Ions and salts
 An ''ion'' is a charged species, an atom or a molecule, that has lost or gained one or more electrons. When an atom loses an electron and thus has more protons than electrons, the atom is a positively charged ion or
An ''ion'' is a charged species, an atom or a molecule, that has lost or gained one or more electrons. When an atom loses an electron and thus has more protons than electrons, the atom is a positively charged ion or cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. When an atom gains an electron and thus has more electrons than protons, the atom is a negatively charged ion or anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. Cations and anions can form a crystalline lattice of neutral salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively cha ...
, such as the Na+ and Cl− ions forming sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, or NaCl. Examples of polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ...
s that do not split up during acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica ...
s are hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
(OH−) and phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
(PO43−).
Plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
is composed of gaseous matter that has been completely ionized, usually through high temperature.
Acidity and basicity
Arrhenius theory Arrhenius may refer to
* Birgit Arrhenius (born 1932), Swedish archaeologist
* Carl Axel Arrhenius (1757–1824), Swedish army lieutenant and amateur mineralogist who discovered ytterbite, a mineral that led to the discovery of yttrium by Johan Gado ...
, which states that acid is a substance that produces hydronium ion
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is d ...
s when it is dissolved in water, and a base is one that produces hydroxide ions when dissolved in water. According to Brønsted–Lowry acid–base theory
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923. The fundamental concept of this theory ...
, acids are substances that donate a positive hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
to another substance in a chemical reaction; by extension, a base is the substance which receives that hydrogen ion.
A third common theory is Lewis acid–base theory
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty Non-bonding orbital, orbital which is capable of accepting an electron pair from a Lewis Base (chemistry), base to form a Lewis a ...
, which is based on the formation of new chemical bonds. Lewis theory explains that an acid is a substance which is capable of accepting a pair of electrons from another substance during the process of bond formation, while a base is a substance which can provide a pair of electrons to form a new bond. There are several other ways in which a substance may be classified as an acid or a base, as is evident in the history of this concept.
Acid strength is commonly measured by two methods. One measurement, based on the Arrhenius definition of acidity, is pH, which is a measurement of the hydronium ion concentration in a solution, as expressed on a negative logarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 o ...
ic scale. Thus, solutions that have a low pH have a high hydronium ion concentration and can be said to be more acidic. The other measurement, based on the Brønsted–Lowry definition, is the acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
(Ka), which measures the relative ability of a substance to act as an acid under the Brønsted–Lowry definition of an acid. That is, substances with a higher Ka are more likely to donate hydrogen ions in chemical reactions than those with lower Ka values.
Redox
Redox (''red''uction-''ox''idation) reactions include allchemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s in which atoms have their oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
changed by either gaining electrons (reduction) or losing electrons (oxidation). Substances that have the ability to oxidize other substances are said to be oxidative and are known as oxidizing agents
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
, oxidants or oxidizers. An oxidant removes electrons from another substance. Similarly, substances that have the ability to reduce other substances are said to be reductive and are known as reducing agents
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth meta ...
, reductants, or reducers.
A reductant transfers electrons to another substance and is thus oxidized itself. And because it "donates" electrons it is also called an electron donor. Oxidation and reduction properly refer to a change in oxidation number—the actual transfer of electrons may never occur. Thus, oxidation is better defined as an increase in oxidation number
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
, and reduction as a decrease in oxidation number.
Equilibrium
Although the concept of equilibrium is widely used across sciences, in the context of chemistry, it arises whenever a number of different states of the chemical composition are possible, as for example, in a mixture of several chemical compounds that can react with one another, or when a substance can be present in more than one kind of phase. A system of chemical substances at equilibrium, even though having an unchanging composition, is most often notstatic
Static may refer to:
Places
*Static Nunatak, a nunatak in Antarctica
United States
* Static, Kentucky and Tennessee
*Static Peak, a mountain in Wyoming
**Static Peak Divide, a mountain pass near the peak
Science and technology Physics
*Static el ...
; molecules of the substances continue to react with one another thus giving rise to a dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances transition between the reactants and products at equal rates, meaning there is no net change. Reactants and products are formed at such a rate that the conc ...
. Thus the concept describes the state in which the parameters such as chemical composition remain unchanged over time.
Chemical laws
Chemical reactions are governed by certain laws, which have become fundamental concepts in chemistry. Some of them are: *Avogadro's law
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific c ...
* Beer–Lambert law
The Beer–Lambert law, also known as Beer's law, the Lambert–Beer law, or the Beer–Lambert–Bouguer law relates the attenuation of light to the properties of the material through which the light is travelling. The law is commonly applied t ...
* Boyle's law
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as:
The ...
(1662, relating pressure and volume)
* Charles's law
Charles's law (also known as the law of volumes) is an experimental gas law that describes how gases tend to expand when heated. A modern statement of Charles's law is:
When the pressure on a sample of a dry gas is held constant, the Kelvin t ...
(1787, relating volume and temperature)
* Fick's laws of diffusion
* Gay-Lussac's law
Gay-Lussac's law usually refers to Joseph-Louis Gay-Lussac's law of combining volumes of gases, discovered in 1808 and published in 1809. It sometimes refers to the proportionality of the volume of a gas to its absolute temperature at constant pr ...
(1809, relating pressure and temperature)
* Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
* Henry's law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulat ...
* Hess's law
* Law of conservation of energy
In physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be ''conserved'' over time. This law, first proposed and tested by Émilie du Châtelet, means that ...
leads to the important concepts of equilibrium, thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
, and kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
.
* Law of conservation of mass continues to be conserved in isolated system
In physical science, an isolated system is either of the following:
# a physical system so far removed from other systems that it does not interact with them.
# a thermodynamic system enclosed by rigid immovable walls through which neither m ...
s, even in modern physics. However, special relativity
In physics, the special theory of relativity, or special relativity for short, is a scientific theory regarding the relationship between space and time. In Albert Einstein's original treatment, the theory is based on two postulates:
# The laws o ...
shows that due to mass–energy equivalence
In physics, mass–energy equivalence is the relationship between mass and energy in a system's rest frame, where the two quantities differ only by a multiplicative constant and the units of measurement. The principle is described by the physicis ...
, whenever non-material "energy" (heat, light, kinetic energy) is removed from a non-isolated system, some mass will be lost with it. High energy losses result in loss of weighable amounts of mass, an important topic in nuclear chemistry
Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
It is the chemistry of radioactive elements such as t ...
.
* Law of definite composition
In chemistry, the law of definite proportions, sometimes called Proust's law, or law of constant composition states that a given
chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source a ...
, although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction.
* Law of multiple proportions
In chemistry, the law of multiple proportions states that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole ...
* Raoult's law
History
The history of chemistry spans a period from very old times to the present. Since several millennia BC, civilizations were using technologies that would eventually form the basis of the various branches of chemistry. Examples include extractingmetal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s from ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
s, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are use ...
, making glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of ...
, and making alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
s like bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
.
Chemistry was preceded by its protoscience, alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
, which operated a non-scientific approach to understanding the constituents of matter and their interactions. Despite being unsuccessful in explaining the nature of matter and its transformations, alchemists set the stage for modern chemistry by performing experiments and recording the results. Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, although skeptical of elements and convinced of alchemy, played a key part in elevating the "sacred art" as an independent, fundamental and philosophical discipline in his work ''The Sceptical Chymist
''The Sceptical Chymist: or Chymico-Physical Doubts & Paradoxes'' is the title of a book by Robert Boyle, published in London in 1661. In the form of a dialogue, the ''Sceptical Chymist'' presented Boyle's hypothesis that matter consisted of corp ...
'' (1661).
While both alchemy and chemistry are concerned with matter and its transformations, the crucial difference was given by the scientific method
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century (with notable practitioners in previous centuries; see the article history of scientific m ...
that chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
s employed in their work. Chemistry, as a body of knowledge distinct from alchemy, became an established science with the work of Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and th ...
, who developed a law of conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass can ...
that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry afterwards is intertwined with the history of thermodynamics
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely wo ...
, especially through the work of Willard Gibbs
Josiah Willard Gibbs (; February 11, 1839 – April 28, 1903) was an American scientist who made significant theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in ...
.
Definition
The definition of chemistry has changed over time, as new discoveries and theories add to the functionality of the science. The term "chymistry", in the view of noted scientistRobert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
in 1661, meant the subject of the material principles of mixed bodies. In 1663, the chemist Christopher Glaser
Christopher Glaser (1615 – between 1670 and 1678), a pharmaceutical chemist of the 17th century.
Life
He was born in Basel. He became demonstrator of chemistry, as successor of Lefebvre, at the Jardin du Roi in Paris, and apothecary to Louis ...
described "chymistry" as a scientific art, by which one learns to dissolve bodies, and draw from them the different substances on their composition, and how to unite them again, and exalt them to a higher perfection.
The 1730 definition of the word "chemistry", as used by Georg Ernst Stahl
Georg Ernst Stahl (22 October 1659 – 24 May 1734) was a German chemist, physician and philosopher. He was a supporter of vitalism, and until the late 18th century his works on phlogiston were accepted as an explanation for chemical processes.K ...
, meant the art of resolving mixed, compound, or aggregate bodies into their principles; and of composing such bodies from those principles. In 1837, Jean-Baptiste Dumas
Jean Baptiste André Dumas (14 July 180010 April 1884) was a French chemist, best known for his works on organic analysis and synthesis, as well as the determination of atomic weights (relative atomic masses) and molecular weights by measuring v ...
considered the word "chemistry" to refer to the science concerned with the laws and effects of molecular forces. This definition further evolved until, in 1947, it came to mean the science of substances: their structure, their properties, and the reactions that change them into other substances – a characterization accepted by Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific top ...
. More recently, in 1998, Professor Raymond Chang broadened the definition of "chemistry" to mean the study of matter and the changes it undergoes.
Background
 Early civilizations, such as the
Early civilizations, such as the Egyptians
Egyptians ( arz, المَصرِيُون, translit=al-Maṣriyyūn, ; arz, المَصرِيِين, translit=al-Maṣriyyīn, ; cop, ⲣⲉⲙⲛ̀ⲭⲏⲙⲓ, remenkhēmi) are an ethnic group native to the Nile, Nile Valley in Egypt. Egyptian ...
Babylonians
Babylonia (; Akkadian: , ''māt Akkadī'') was an ancient Akkadian-speaking state and cultural area based in the city of Babylon in central-southern Mesopotamia (present-day Iraq and parts of Syria). It emerged as an Amorite-ruled state c. ...
and Indians amassed practical knowledge concerning the arts of metallurgy, pottery and dyes, but didn't develop a systematic theory.
A basic chemical hypothesis first emerged in Classical Greece
Classical Greece was a period of around 200 years (the 5th and 4th centuries BC) in Ancient Greece,The "Classical Age" is "the modern designation of the period from about 500 B.C. to the death of Alexander the Great in 323 B.C." ( Thomas R. Marti ...
with the theory of four elements
Classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Tibet, and India had simi ...
as propounded definitively by Aristotle
Aristotle (; grc-gre, Ἀριστοτέλης ''Aristotélēs'', ; 384–322 BC) was a Greek philosopher and polymath during the Classical period in Ancient Greece. Taught by Plato, he was the founder of the Peripatetic school of phil ...
stating that fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
At a certain point in the combustion reaction, called the ignition ...
, air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
, earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
were the fundamental elements from which everything is formed as a combination. Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
atomism
Atomism (from Greek , ''atomon'', i.e. "uncuttable, indivisible") is a natural philosophy proposing that the physical universe is composed of fundamental indivisible components known as atoms.
References to the concept of atomism and its atoms ...
dates back to 440 BC, arising in works by philosophers such as Democritus
Democritus (; el, Δημόκριτος, ''Dēmókritos'', meaning "chosen of the people"; – ) was an Ancient Greek pre-Socratic philosopher from Abdera, primarily remembered today for his formulation of an atomic theory of the universe. No ...
and Epicurus
Epicurus (; grc-gre, Ἐπίκουρος ; 341–270 BC) was an ancient Greek philosopher and sage who founded Epicureanism, a highly influential school of philosophy. He was born on the Greek island of Samos to Athenian parents. Influenced ...
. In 50 BCE, the Roman
Roman or Romans most often refers to:
*Rome, the capital city of Italy
*Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of ancient Rome
*''Epistle to the Romans'', shortened to ''Romans'', a letter ...
philosopher Lucretius
Titus Lucretius Carus ( , ; – ) was a Roman poet and philosopher. His only known work is the philosophical poem ''De rerum natura'', a didactic work about the tenets and philosophy of Epicureanism, and which usually is translated into E ...
expanded upon the theory in his book ''De rerum natura
''De rerum natura'' (; ''On the Nature of Things'') is a first-century BC didactic poem by the Roman poet and philosopher Lucretius ( – c. 55 BC) with the goal of explaining Epicurean philosophy to a Roman audience. The poem, written in some 7 ...
'' (On The Nature of Things). Unlike modern concepts of science, Greek atomism was purely philosophical in nature, with little concern for empirical observations and no concern for chemical experiments.
An early form of the idea of conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass can ...
is the notion that "Nothing comes from nothing
Nothing comes from nothing ( gr, οὐδὲν ἐξ οὐδενός; la, ex nihilo nihil fit) is a philosophical dictum first argued by Parmenides. It is associated with ancient Greek cosmology, such as is presented not just in the works of Homer ...
" in Ancient Greek philosophy
Ancient Greek philosophy arose in the 6th century BC, marking the end of the Greek Dark Ages. Greek philosophy continued throughout the Hellenistic period and the period in which Greece and most Greek-inhabited lands were part of the Roman Empire ...
, which can be found in Empedocles
Empedocles (; grc-gre, Ἐμπεδοκλῆς; , 444–443 BC) was a Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is best known for originating the cosmogonic theory of the fo ...
(approx. 4th century BC): "For it is impossible for anything to come to be from what is not, and it cannot be brought about or heard of that what is should be utterly destroyed." and Epicurus
Epicurus (; grc-gre, Ἐπίκουρος ; 341–270 BC) was an ancient Greek philosopher and sage who founded Epicureanism, a highly influential school of philosophy. He was born on the Greek island of Samos to Athenian parents. Influenced ...
(3rd century BC), who, describing the nature of the Universe, wrote that "the totality of things was always such as it is now, and always will be".
 In the
In the Hellenistic world
In Classical antiquity, the Hellenistic period covers the time in Mediterranean history after Classical Greece, between the death of Alexander the Great in 323 BC and the emergence of the Roman Empire, as signified by the Battle of Actium in 3 ...
the art of alchemy first proliferated, mingling magic and occultism into the study of natural substances with the ultimate goal of transmuting elements into gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile met ...
and discovering the elixir of eternal life. Work, particularly the development of distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
, continued in the early Byzantine
The Byzantine Empire, also referred to as the Eastern Roman Empire or Byzantium, was the continuation of the Roman Empire primarily in its eastern provinces during Late Antiquity and the Middle Ages, when its capital city was Constantinopl ...
period with the most famous practitioner being the 4th century Greek-Egyptian Zosimos of Panopolis
Zosimos of Panopolis ( el, Ζώσιμος ὁ Πανοπολίτης; also known by the Latin name Zosimus Alchemista, i.e. "Zosimus the Alchemist") was a Greco-Egyptian alchemist and Gnostic mystic who lived at the end of the 3rd and beginning ...
. Alchemy continued to be developed and practised throughout the Arab world
The Arab world ( ar, اَلْعَالَمُ الْعَرَبِيُّ '), formally the Arab homeland ( '), also known as the Arab nation ( '), the Arabsphere, or the Arab states, refers to a vast group of countries, mainly located in Western A ...
after the Muslim conquests
The early Muslim conquests or early Islamic conquests ( ar, الْفُتُوحَاتُ الإسْلَامِيَّة, ), also referred to as the Arab conquests, were initiated in the 7th century by Muhammad, the main Islamic prophet. He estab ...
, and from there, and from the Byzantine remnants, diffused into medieval and Renaissance
The Renaissance ( , ) , from , with the same meanings. is a period in European history marking the transition from the Middle Ages to modernity and covering the 15th and 16th centuries, characterized by an effort to revive and surpass ideas ...
Europe through Latin translations.
The Arabic works attributed to Jabir ibn Hayyan
Abū Mūsā Jābir ibn Ḥayyān (Arabic: , variously called al-Ṣūfī, al-Azdī, al-Kūfī, or al-Ṭūsī), died 806−816, is the purported author of an enormous number and variety of works in Arabic, often called the Jabirian corpus. The ...
introduced a systematic classification of chemical substances, and provided instructions for deriving an inorganic compound (sal ammoniac
Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is ...
or ammonium chloride) from organic substances (such as plants, blood, and hair) by chemical means. Some Arabic Jabirian works (e.g., the "Book of Mercy", and the "Book of Seventy") were later translated into Latin under the Latinized name "Geber", and in 13th-century Europe an anonymous writer, usually referred to as pseudo-Geber
Pseudo-Geber (or "Latin pseudo-Geber") is the presumed author or group of authors responsible for a corpus of pseudepigraphic alchemical writings dating to the late 13th and early 14th centuries. These writings were falsely attributed to Jabir ...
, started to produce alchemical and metallurgical writings under this name. Later influential Muslim philosophers, such as Abū al-Rayhān al-Bīrūnī
Abu Rayhan Muhammad ibn Ahmad al-Biruni (973 – after 1050) commonly known as al-Biruni, was a Khwarazmian Iranian in scholar and polymath during the Islamic Golden Age. He has been called variously the "founder of Indology", "Father of Co ...
and Avicenna
Ibn Sina ( fa, ابن سینا; 980 – June 1037 CE), commonly known in the West as Avicenna (), was a Persian polymath who is regarded as one of the most significant physicians, astronomers, philosophers, and writers of the Islamic G ...
disputed the theories of alchemy, particularly the theory of the transmutation of metals.
Under the influence of the new empirical methods propounded by Sir Francis Bacon
Francis Bacon, 1st Viscount St Alban (; 22 January 1561 – 9 April 1626), also known as Lord Verulam, was an English philosopher and statesman who served as Attorney General and Lord Chancellor of England. Bacon led the advancement of both n ...
and others, a group of chemists at Oxford
Oxford () is a city in England. It is the county town and only city of Oxfordshire. In 2020, its population was estimated at 151,584. It is north-west of London, south-east of Birmingham and north-east of Bristol. The city is home to the ...
, Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, Robert Hooke
Robert Hooke FRS (; 18 July 16353 March 1703) was an English polymath active as a scientist, natural philosopher and architect, who is credited to be one of two scientists to discover microorganisms in 1665 using a compound microscope that ...
and John Mayow
John Mayow FRS (1641–1679) was a chemist, physician, and physiologist who is remembered today for conducting early research into respiration and the nature of air. Mayow worked in a field that is sometimes called pneumatic chemistry.
Lif ...
began to reshape the old alchemical traditions into a scientific discipline. Boyle in particular questioned some commonly held chemical theories and argued for chemical practitioners to be more "philosophical" and less commercially focused in '' The Sceptical Chemyst''. He formulated Boyle's law
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as:
The ...
, rejected the classical "four elements" and proposed a mechanistic alternative of atoms and chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s that could be subject to rigorous experiment.
 In the following decades, many important discoveries were made, such as the nature of 'air' which was discovered to be composed of many different gases. The Scottish chemist
In the following decades, many important discoveries were made, such as the nature of 'air' which was discovered to be composed of many different gases. The Scottish chemist Joseph Black
Joseph Black (16 April 1728 – 6 December 1799) was a Scottish physicist and chemist, known for his discoveries of magnesium, latent heat, specific heat, and carbon dioxide. He was Professor of Anatomy and Chemistry at the University of Glas ...
and the Flemish Jan Baptist van Helmont
Jan Baptist van Helmont (; ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered to ...
discovered carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
, or what Black called 'fixed air' in 1754; Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
discovered hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and elucidated its properties and Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted exp ...
and, independently, Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydrog ...
isolated pure oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. The theory of phlogiston
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burni ...
(a substance at the root of all combustion) was propounded by the German Georg Ernst Stahl
Georg Ernst Stahl (22 October 1659 – 24 May 1734) was a German chemist, physician and philosopher. He was a supporter of vitalism, and until the late 18th century his works on phlogiston were accepted as an explanation for chemical processes.K ...
in the early 18th century and was only overturned by the end of the century by the French chemist Antoine Lavoisier
Antoine-Laurent de Lavoisier ( , ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and th ...
, the chemical analogue of Newton in physics. Lavoisier did more than any other to establish the new science on proper theoretical footing, by elucidating the principle of conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass can ...
and developing a new system of chemical nomenclature used to this day.
English scientist John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
proposed the modern theory of atoms; that all substances are composed of indivisible 'atoms' of matter and that different atoms have varying atomic weights.
The development of the electrochemical theory of chemical combinations occurred in the early 19th century as the result of the work of two scientists in particular, Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be on ...
and Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
, made possible by the prior invention of the voltaic pile
upright=1.2, Schematic diagram of a copper–zinc voltaic pile. The copper and zinc discs were separated by cardboard or felt spacers soaked in salt water (the electrolyte). Volta's original piles contained an additional zinc disk at the bottom, ...
by Alessandro Volta
Alessandro Giuseppe Antonio Anastasio Volta (, ; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist and lay Catholic who was a pioneer of electricity and power who is credited as the inventor of the electric battery and the ...
. Davy discovered nine new elements including the alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
by extracting them from their oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s with electric current.
British William Prout
William Prout FRS (; 15 January 1785 – 9 April 1850) was an English chemist, physician, and natural theologian. He is remembered today mainly for what is called Prout's hypothesis.
Biography
Prout was born in Horton, Gloucestershire in 1 ...
first proposed ordering all the elements by their atomic weight as all atoms had a weight that was an exact multiple of the atomic weight of hydrogen. J.A.R. Newlands
John Alexander Reina Newlands (26 November 1837 – 29 July 1898) was a British chemist who worked concerning the periodicity of elements.
Biography
Newlands was born in London in England, at West Square in Lambeth, the son of a Scottish Pre ...
devised an early table of elements, which was then developed into the modern periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
of elements in the 1860s by Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
and independently by several other scientists including Julius Lothar Meyer
Julius Lothar Meyer (19 August 1830 – 11 April 1895) was a German chemist. He was one of the pioneers in developing the earliest versions of the periodic table of the chemical elements. Russian chemist Dmitri Mendeleev (his chief rival) and he ...
. The inert gases, later called the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
es were discovered by William Ramsay
Sir William Ramsay (; 2 October 1852 – 23 July 1916) was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous element ...
in collaboration with Lord Rayleigh
John William Strutt, 3rd Baron Rayleigh, (; 12 November 1842 – 30 June 1919) was an English mathematician and physicist who made extensive contributions to science. He spent all of his academic career at the University of Cambridge. Amo ...
at the end of the century, thereby filling in the basic structure of the table.
J.J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
of the University of Cambridge
, mottoeng = Literal: From here, light and sacred draughts.
Non literal: From this place, we gain enlightenment and precious knowledge.
, established =
, other_name = The Chancellor, Masters and Schola ...
discovered the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
and soon after the French scientist Becquerel as well as the couple Pierre Curie, Pierre and Marie Curie investigated the phenomenon of radioactivity. In a series of pioneering scattering experiments Ernest Rutherford at the University of Manchester discovered the internal structure of the atom and the existence of the proton, classified and explained the different types of radioactivity and successfully Nuclear transmutation, transmuted the first element by bombarding nitrogen with alpha particles.
His work on atomic structure was improved on by his students, the Danish physicist Niels Bohr, the Englishman Henry Moseley and the German Otto Hahn, who went on to father the emerging nuclear chemistry
Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
It is the chemistry of radioactive elements such as t ...
and discovered nuclear fission. The electronic theory of chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s and molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s was developed by the American scientists Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific top ...
and Gilbert N. Lewis.
The year 2011 was declared by the United Nations as the International Year of Chemistry. It was an initiative of the International Union of Pure and Applied Chemistry, and of the United Nations Educational, Scientific, and Cultural Organization and involves chemical societies, academics, and institutions worldwide and relied on individual initiatives to organize local and regional activities.
Organic chemistry was developed by Justus von Liebig and others, following Friedrich Wöhler's synthesis of urea. Other crucial 19th century advances were; an understanding of valence bonding (Edward Frankland in 1852) and the application of thermodynamics to chemistry (J. W. Gibbs and Svante Arrhenius in the 1870s).
Practice
Subdisciplines
Chemistry is typically divided into several major sub-disciplines. There are also several main cross-disciplinary and more specialized fields of chemistry. * Analytical chemistry is the analysis of material samples to gain an understanding of theirchemical composition
A chemical composition specifies the identity, arrangement, and ratio of the elements making up a compound.
Chemical formulas can be used to describe the relative amounts of elements present in a compound. For example, the chemical formula for ...
and Chemical structure, structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdisciplines of chemistry, excluding purely theoretical chemistry.
* Biochemistry is the study of the chemical compound, chemicals, chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
* Inorganic chemistry is the study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry.
* Materials Chemistry, Materials chemistry is the preparation, characterization, and understanding of substances with a useful function. The field is a new breadth of study in graduate programs, and it integrates elements from all classical areas of chemistry with a focus on fundamental issues that are unique to materials. Primary systems of study include the chemistry of condensed phases (solids, liquids, polymers) and interface (chemistry), interfaces between different phases.
* Neurochemistry is the study of neurochemicals; including transmitters, peptides, proteins, lipids, sugars, and nucleic acids; their interactions, and the roles they play in forming, maintaining, and modifying the nervous system.
* Nuclear chemistry is the study of how subatomic particles come together and make nuclei. Modern Nuclear transmutation, Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field.
* Organic chemistry is the study of the structure, properties, composition, mechanisms, and chemical reaction, reactions of organic compounds. An organic compound is defined as any compound based on a carbon skeleton.
* Physical chemistry is the study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurement ...
, chemical kinetics, electrochemistry, statistical mechanics, spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
, and more recently, astrochemistry. Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of calculus, infinitesimal calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry. Physical chemistry is a distinct discipline from chemical physics, but again, there is very strong overlap.
* Theoretical chemistry is the study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
). In particular the application of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
to chemistry is called quantum chemistry. Since the end of the World War II, Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics.
Others subdivisions include electrochemistry, femtochemistry, Flavoring, flavor chemistry, flow chemistry, immunohistochemistry, hydrogenation, hydrogenation chemistry, mathematical chemistry, molecular mechanics, natural product chemistry, organometallic chemistry, petrochemistry, photochemistry, physical organic chemistry, polymer chemistry, radiochemistry, sonochemistry, supramolecular chemistry, synthetic chemistry, and many others.
Interdisciplinary
Interdisciplinarity, Interdisciplinary fields include agrochemistry, astrochemistry (andcosmochemistry
Cosmochemistry (from Greek κόσμος ''kósmos'', "universe" and χημεία ''khemeía'') or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is done ...
), atmospheric chemistry, chemical engineering, chemical biology, chemo-informatics, environmental chemistry, geochemistry, green chemistry, immunochemistry, marine chemistry, materials science, mechanochemistry, medicinal chemistry, molecular biology, nanotechnology, oenology, pharmacology
Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemica ...
, phytochemistry, solid-state chemistry, surface science, thermochemistry, and many others.
Industry
The chemical industry represents an important economic activity worldwide. The List of largest chemical producers, global top 50 chemical producers in 2013 had sales of United States dollar, US$980.5 billion with a profit margin of 10.3%.Professional societies
* American Chemical Society * American Society for Neurochemistry * Chemical Institute of Canada * Chemical Society of Peru *International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
* Royal Australian Chemical Institute
* Royal Netherlands Chemical Society
* Royal Society of Chemistry
* Society of Chemical Industry
* World Association of Theoretical and Computational Chemists
* List of chemistry societies
See also
* Comparison of software for molecular mechanics modeling * Glossary of chemistry terms * International Year of Chemistry * List of chemists * List of compounds * List of important publications in chemistry * List of unsolved problems in chemistry * Outline of chemistry * Periodic systems of small molecules * Philosophy of chemistry * Science tourismReferences
Bibliography
* * *Further reading
Popular reading * Atkins, P.W. ''Galileo's Finger'' (Oxford University Press) * Atkins, P.W. ''Atkins' Molecules'' (Cambridge University Press) * Kean, Sam. ''The Disappearing Spoon – and Other True Tales from the Periodic Table'' (Black Swan) London, 2010 * Primo Levi, Levi, Primo ''The Periodic Table'' (Penguin Books) [1975] translated from the Italian by Raymond Rosenthal (1984) * Stwertka, A. ''A Guide to the Elements'' (Oxford University Press) * * Introductory undergraduate textbooks * Atkins, P.W., Overton, T., Rourke, J., Weller, M. and Armstrong, F. ''Shriver and Atkins Inorganic Chemistry'' (4th edition) 2006 (Oxford University Press) * Chang, Raymond. ''Chemistry'' 6th ed. Boston: James M. Smith, 1998. . * * Voet and Voet. ''Biochemistry'' (Wiley) Advanced undergraduate-level or graduate textbooks * Atkins, P. W. ''Physical Chemistry'' (Oxford University Press) * Atkins, P. W. et al. ''Molecular Quantum Mechanics'' (Oxford University Press) * McWeeny, R. ''Coulson's Valence'' (Oxford Science Publications) * Pauling, L. ''The Nature of the chemical bond'' (Cornell University Press) * Pauling, L., and Wilson, E.B. ''Introduction to Quantum Mechanics with Applications to Chemistry'' (Dover Publications) * Smart and Moore. ''Solid State Chemistry: An Introduction'' (Chapman and Hall) * Stephenson, G. ''Mathematical Methods for Science Students'' (Longman)External links
General Chemistry principles, patterns and applications
{{Authority control Chemistry,