Western blot on:
[Wikipedia]
[Google]
[Amazon]
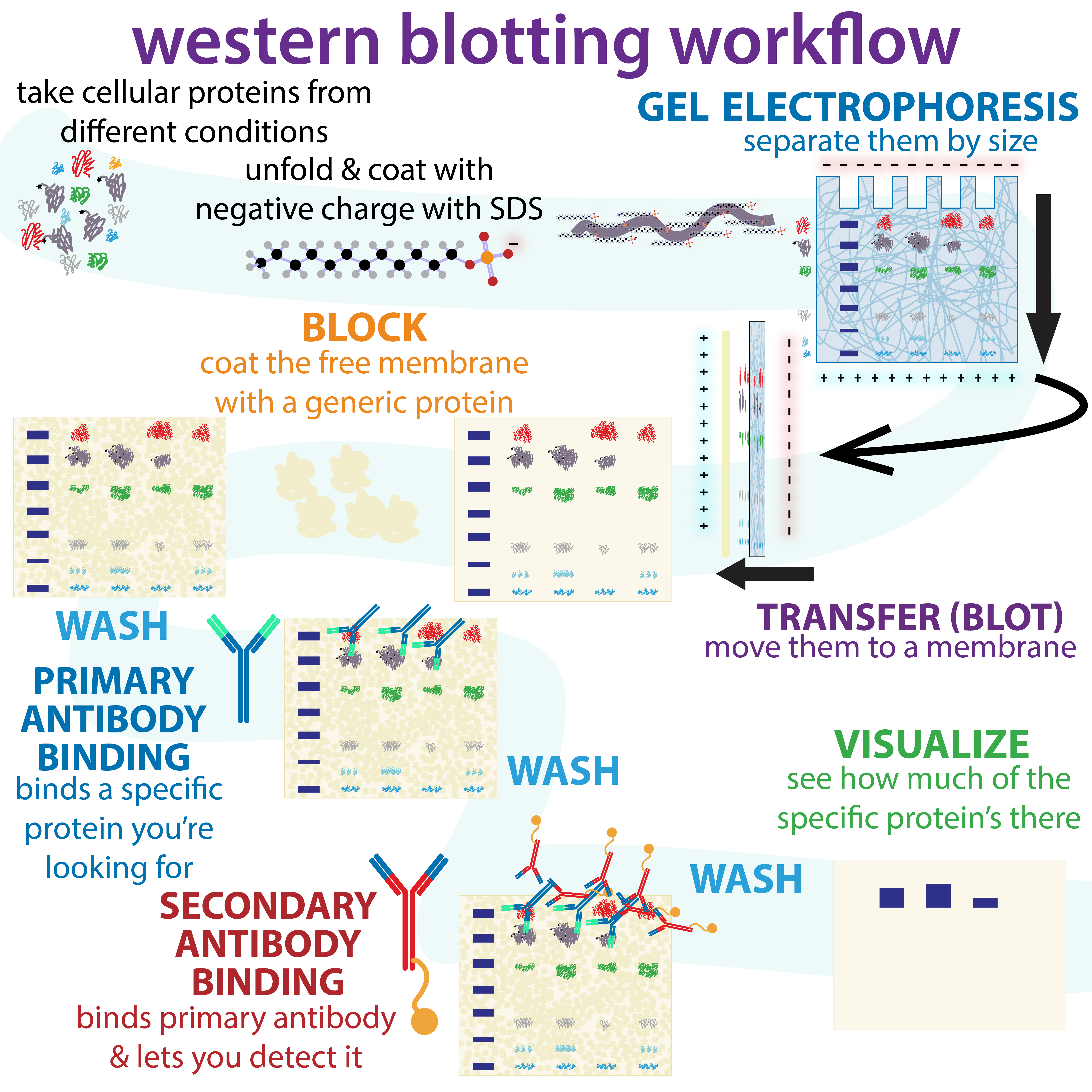 The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in
 The western blot is extensively used in
The western blot is extensively used in
 The proteins of the sample are separated using
The proteins of the sample are separated using
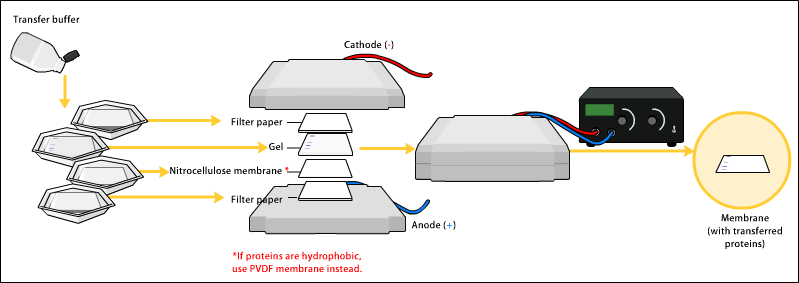 To make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane, a solid support, which is an essential part of the process. There are two types of membrane: '' nitrocellulose (NC) or polyvinylidene difluoride (PVDF''). NC membrane has high affinity for protein and its retention abilities. However, NC is brittle, and does not allow the blot to be used for re-probing, whereas PVDF membrane allows the blot to be re-probed. The most commonly used method for transferring the proteins is called
To make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane, a solid support, which is an essential part of the process. There are two types of membrane: '' nitrocellulose (NC) or polyvinylidene difluoride (PVDF''). NC membrane has high affinity for protein and its retention abilities. However, NC is brittle, and does not allow the blot to be used for re-probing, whereas PVDF membrane allows the blot to be re-probed. The most commonly used method for transferring the proteins is called
 Total protein staining allows the total protein that has been successfully transferred to the membrane to be visualised, allowing the user to check the uniformity of protein transfer and to perform subsequent normalization of the target protein with the actual protein amount per lane. Normalization with the so-called "loading control" was based on immunostaining of housekeeping proteins in the classical procedure, but is heading toward total protein staining recently, due to multiple benefits. At least seven different approaches for total protein staining have been described for western blot normalization: Ponceau S, stain-free techniques, Sypro Ruby, Epicocconone, Coomassie R-350, Amido Black, and Cy5. In order to avoid noise of signal, total protein staining should be performed before blocking of the membrane. Nevertheless, post-antibody stainings have been described as well.
Total protein staining allows the total protein that has been successfully transferred to the membrane to be visualised, allowing the user to check the uniformity of protein transfer and to perform subsequent normalization of the target protein with the actual protein amount per lane. Normalization with the so-called "loading control" was based on immunostaining of housekeeping proteins in the classical procedure, but is heading toward total protein staining recently, due to multiple benefits. At least seven different approaches for total protein staining have been described for western blot normalization: Ponceau S, stain-free techniques, Sypro Ruby, Epicocconone, Coomassie R-350, Amido Black, and Cy5. In order to avoid noise of signal, total protein staining should be performed before blocking of the membrane. Nevertheless, post-antibody stainings have been described as well.
 During the detection process, the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme; when exposed to an appropriate substrate, this enzyme drives a colorimetric reaction and produces a color. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
During the detection process, the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme; when exposed to an appropriate substrate, this enzyme drives a colorimetric reaction and produces a color. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
 After the unbound probes are washed away, the western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is commonly repeated for a structural protein, such as
After the unbound probes are washed away, the western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is commonly repeated for a structural protein, such as
 The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as a CCD camera equipped with appropriate emission filters which captures a digital image of the western blot and allows further data analysis such as molecular weight analysis and a quantitative western blot analysis. Fluorescence is considered to be one of the best methods for quantification but is less sensitive than chemiluminescence.
The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as a CCD camera equipped with appropriate emission filters which captures a digital image of the western blot and allows further data analysis such as molecular weight analysis and a quantitative western blot analysis. Fluorescence is considered to be one of the best methods for quantification but is less sensitive than chemiluminescence.
Sciugo
Ghostarchive
and th
Wayback Machine
* Archived a
Ghostarchive
and th
Wayback Machine
{{Molecular probes Diagnostic virology Protein methods Molecular biology Laboratory techniques Molecular biology techniques
 The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and phys ...
and immunogenetics
Immunogenetics or immungenetics is the branch of Medical Immunology and Medical Genetics that explores the relationship between the immune system and genetics.
Autoimmune diseases, such as type 1 diabetes, are complex genetic traits which res ...
to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination.
Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic Synthetic things are composed of multiple parts, often with the implication that they are artificial. In particular, 'synthetic' may refer to:
Science
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic o ...
or animal-derived antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and Viral disease, viruses. The antibody recognizes a unique m ...
(known as the primary antibody
Primary and secondary antibodies are two groups of antibodies that are classified based on whether they bind to ''antigens or proteins'' directly or target another (primary) antibody that, in turn, is bound to an ''antigen or protein''.
Primary
A ...
) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibody. The secondary antibody is visualized through various methods such as staining
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in ...
, immunofluorescence, and radioactivity, allowing indirect detection of the specific target protein.
Other related techniques include dot blot analysis, quantitative dot blot
A dot blot (or slot blot) is a technique in molecular biology used to detect proteins. It represents a simplification of the western blot method, with the exception that the proteins to be detected are not first separated by electrophoresis. Instea ...
, immunohistochemistry and immunocytochemistry
Immunocytochemistry (ICC) is a common laboratory technique that is used to anatomically visualize the localization of a specific protein or antigen in cells by use of a specific primary antibody that binds to it. The primary antibody allows visua ...
, where antibodies are used to detect proteins in tissues and cells by immunostaining, and enzyme-linked immunosorbent assay (ELISA).
The name ''western blot'' is a play on the Southern blot
A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detec ...
, a technique for DNA detection named after its inventor, English biologist Edwin Southern
Sir Edwin Mellor Southern (born 7 June 1938) is an English Lasker Award-winning molecular biologist, Emeritus Professor of Biochemistry at the University of Oxford and a fellow of Trinity College, Oxford. He is most widely known for the inve ...
. Similarly, detection of RNA is termed as northern blot
The northern blot, or RNA blot,Gilbert, S. F. (2000) Developmental Biology, 6th Ed. Sunderland MA, Sinauer Associates. is a technique used in molecular biology research to study gene expression by detection of RNA (or isolated mRNA) in a sample.Ke ...
. The term "western blot" was given by W. Neal Burnette in 1981, although the method itself was independently invented in 1979 by
Jaime Renart, Jakob Reiser, and George Stark in Stanford
Stanford University, officially Leland Stanford Junior University, is a private research university in Stanford, California. The campus occupies , among the largest in the United States, and enrolls over 17,000 students. Stanford is consider ...
, and by Harry Towbin, Theophil Staehelin, and Julian Gordon at the Friedrich Miescher Institute
The Friedrich Miescher Institute for Biomedical Research (FMI) is a biomedical research institute founded in 1970. Based in Basel, Switzerland, the FMI is affiliated with the University of Basel and the Novartis Institutes for BioMedical Resear ...
in Basel
, french: link=no, Bâlois(e), it, Basilese
, neighboring_municipalities= Allschwil (BL), Hégenheim (FR-68), Binningen (BL), Birsfelden (BL), Bottmingen (BL), Huningue (FR-68), Münchenstein (BL), Muttenz (BL), Reinach (BL), Riehen (BS) ...
, Switzerland. Between 1979 and 2019 "it has been mentioned in the titles, abstracts, and keywords of more than 400,000 PubMed
PubMed is a free search engine accessing primarily the MEDLINE database of references and abstracts on life sciences and biomedical topics. The United States National Library of Medicine (NLM) at the National Institutes of Health maintain ...
-listed publications" and may still be the most used protein-analytical technique.
Applications
 The western blot is extensively used in
The western blot is extensively used in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology ...
for the qualitative detection of single proteins and protein-modifications (such as post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribos ...
s). At least 8–9% of all protein-related publications are estimated to apply western blots. It is used as a general method to identify the presence of a specific single protein within a complex mixture of proteins. A semi-quantitative estimation of a protein can be derived from the size and color intensity of a protein band on the blot membrane. In addition, applying a dilution series of a purified protein of known concentrations can be used to allow a more precise estimate of protein concentration. The western blot is routinely used for verification of protein production after cloning
Cloning is the process of producing individual organisms with identical or virtually identical DNA, either by natural or artificial means. In nature, some organisms produce clones through asexual reproduction. In the field of biotechnology, c ...
. It is also used in medical diagnostics, e.g., in the HIV test or BSE-Test.
The confirmatory HIV test employs a western blot to detect anti-HIV antibody in a human serum
Serum may refer to:
*Serum (blood), plasma from which the clotting proteins have been removed
**Antiserum, blood serum with specific antibodies for passive immunity
* Serous fluid, any clear bodily fluid
* Truth serum, a drug that is likely to mak ...
sample. Proteins from known HIV
The human immunodeficiency viruses (HIV) are two species of '' Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immu ...
-infected cells are separated and blotted on a membrane as above. Then, the serum to be tested is applied in the primary antibody incubation step; free antibody is washed away, and a secondary anti-human antibody linked to an enzyme signal is added. The stained bands then indicate the proteins to which the patient's serum contains antibody. A western blot is also used as the definitive test for variant Creutzfeldt–Jakob Disease, a type of prion disease linked to the consumption of contaminated beef from cattle with bovine spongiform encephalopathy (BSE, commonly referred to as 'mad cow disease'). Another application is in the diagnosis of tularemia. An evaluation of the western blot's ability to detect antibodies against ''F. tularensis
''Francisella tularensis'' is a pathogenic species of Gram-negative coccobacillus, an aerobic bacterium. It is nonspore-forming, nonmotile, and the causative agent of tularemia, the pneumonic form of which is often lethal without treatment. It ...
'' revealed that its sensitivity is almost 100% and the specificity is 99.6%. Some forms of Lyme disease testing employ western blotting. A western blot can also be used as a confirmatory test for Hepatitis B infection and HSV-2 (Herpes Type 2) infection. In veterinary medicine, a western blot is sometimes used to confirm FIV+ status in cats.
Further applications of the western blot technique include its use by the World Anti-Doping Agency (WADA). Blood doping is the misuse of certain techniques and/or substances to increase one's red blood cell mass, which allows the body to transport more oxygen to muscles and therefore increase stamina and performance. There are three widely known substances or methods used for blood doping, namely, erythropoietin
Erythropoietin (; EPO), also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bon ...
(EPO), synthetic oxygen carriers and blood transfusions. Each is prohibited under WADA's List of Prohibited Substances and Methods. The western blot technique was used during the 2014 FIFA World Cup in the anti-doping campaign for that event. In total, over 1000 samples were collected and analyzed by Reichel, et al. in the WADA accredited Laboratory of Lausanne, Switzerland. Recent research utilizing the western blot technique showed an improved detection of EPO in blood and urine based on novel Velum SAR precast horizontal gels optimized for routine analysis. With the adoption of the horizontal SAR-PAGE in combination with the precast film-supported Velum SAR gels the discriminatory capacity of micro-dose application of rEPO was significantly enhanced.
Procedure
The western blot method is composed of agel electrophoresis
Gel electrophoresis is a method for separation and analysis of biomacromolecules ( DNA, RNA, proteins, etc.) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size (IEF ...
to separate native proteins by 3-D structure or denatured proteins by the length of the polypeptide, followed by an electrophoretic transfer onto a membrane (mostly PVDF or nitrocellulose) and an immunostaining procedure to visualize a certain protein on the blot membrane. SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. ...
is generally used for the denaturing electrophoretic separation of proteins. SDS is generally used as a buffer (as well as in the gel) in order to give all proteins present a uniform negative charge, since proteins can be positively, negatively, or neutrally charged. This type of electrophoresis is known as SDS-PAGE (SDS-polyacrylamide gel electrophoresis). Prior to electrophoresis, protein samples are often boiled to denature the proteins present. This ensures that proteins are separated based on size and prevents proteases
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the ...
(enzymes that break down proteins) from degrading samples. Following electrophoretic separation, the proteins are transferred to a membrane (typically nitrocellulose or PVDF). The membrane is often then stained with Ponceau S in order to visualize the proteins on the blot and ensure a proper transfer occurred. Next the proteins are blocked with milk (or other blocking agents) to prevent non-specific antibody binding, and then stained with antibodies specific to the target protein. Lastly, the membrane will be stained with a secondary antibody that recognizes the first antibody staining, which can then be used for detection by a variety of methods. The gel electrophoresis step is included in western blot analysis to resolve the issue of the cross-reactivity of antibodies.
Sample Preparation
As a significant step in conducting a western blot, sample preparation has to be done effectively since the interpretation of this essay is influenced by the protein preparation, which is composed of protein extraction and purification processes. To achieve efficient protein extraction, a proper homogenization method needs to be chosen due to the fact that it is responsible for bursting the cell membrane and releasing the intracellular components. Besides that, the ideal lysis buffer is needed to acquire substantial amounts of target protein content because the buffer is leading the process of protein solubilization and preventing protein degradation. After completing the sample preparation, the protein content is ready to be separated by the utilization of gel electrophoresis.Gel electrophoresis
 The proteins of the sample are separated using
The proteins of the sample are separated using gel electrophoresis
Gel electrophoresis is a method for separation and analysis of biomacromolecules ( DNA, RNA, proteins, etc.) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size (IEF ...
. Separation of proteins may be by isoelectric point (pI), molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
, electric charge, or a combination of these factors. The nature of the separation depends on the treatment of the sample and the nature of the gel.
By far the most common type of gel electrophoresis employs polyacrylamide
Polyacrylamide (abbreviated as PAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly f ...
gels and buffers loaded with sodium dodecyl sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium sal ...
(SDS). SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. ...
(SDS-polyacrylamide gel electrophoresis) maintains polypeptides in a denatured state once they have been treated with strong reducing agents to remove secondary and tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
(e.g. disulfide bonds -Sto sulfhydryl groups H and SH
H, or h, is the eighth letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''aitch'' (pronounced , plural ''aitches''), or region ...
and thus allows separation of proteins by their molecular mass
The molecular mass (''m'') is the mass of a given molecule: it is measured in daltons (Da or u). Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The related quant ...
. Sampled proteins become covered in the negatively charged SDS, effectively becoming anionic, and migrate towards the positively charged (higher voltage) anode (usually having a red wire) through the acrylamide mesh of the gel. Smaller proteins migrate faster through this mesh, and the proteins are thus separated according to size (usually measured in kilodaltons, kDa). The concentration of acrylamide determines the resolution of the gel – the greater the acrylamide concentration, the better the resolution of lower molecular weight proteins. The lower the acrylamide concentration, the better the resolution of higher molecular weight proteins. Proteins travel only in one dimension along the gel for most blots.
Samples are loaded into ''wells'' in the gel. One lane is usually reserved for a ''marker'' or ''ladder'', which is a commercially available mixture of proteins of known molecular weights, typically stained so as to form visible, coloured bands. When voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge t ...
is applied along the gel, proteins migrate through it at different speeds dependent on their size. These different rates of advancement (different ''electrophoretic mobilities'') separate into ''bands'' within each ''lane''. Protein bands can then be compared to the ladder bands, allowing estimation of the protein's molecular weight.
It is also possible to use a two-dimensional gel which spreads the proteins from a single sample out in two dimensions. Proteins are separated according to isoelectric point ( pH at which they have a neutral net charge) in the first dimension, and according to their molecular weight in the second dimension.
Transfer
 To make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane, a solid support, which is an essential part of the process. There are two types of membrane: '' nitrocellulose (NC) or polyvinylidene difluoride (PVDF''). NC membrane has high affinity for protein and its retention abilities. However, NC is brittle, and does not allow the blot to be used for re-probing, whereas PVDF membrane allows the blot to be re-probed. The most commonly used method for transferring the proteins is called
To make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane, a solid support, which is an essential part of the process. There are two types of membrane: '' nitrocellulose (NC) or polyvinylidene difluoride (PVDF''). NC membrane has high affinity for protein and its retention abilities. However, NC is brittle, and does not allow the blot to be used for re-probing, whereas PVDF membrane allows the blot to be re-probed. The most commonly used method for transferring the proteins is called electroblotting
Electroblotting is a method in molecular biology/ biochemistry/immunogenetics to transfer proteins or nucleic acids onto a membrane by using PVDF or nitrocellulose, after gel electrophoresis. The protein or nucleic acid can then be further anal ...
. Electroblotting uses an electric current to pull the negatively charged proteins from the gel towards the positively charged anode, and into the PVDF or NC membrane. The proteins move from within the gel onto the membrane while maintaining the organization they had within the gel. An older method of transfer involves placing a membrane on top of the gel, and a stack of filter papers on top of that. The entire stack is placed in a buffer solution which moves up the paper by capillary action
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces li ...
, bringing the proteins with it. In practice this method is not commonly used due to the lengthy procedure time.
As a result of either transfer process, the proteins are exposed on a thin membrane layer for detection. Both varieties of membrane are chosen for their non-specific protein binding properties (i.e. binds all proteins equally well). Protein binding is based upon hydrophobic interactions, as well as charged interactions between the membrane and protein. Nitrocellulose membranes are cheaper than PVDF, but are far more fragile and cannot withstand repeated probings.
Total protein staining
 Total protein staining allows the total protein that has been successfully transferred to the membrane to be visualised, allowing the user to check the uniformity of protein transfer and to perform subsequent normalization of the target protein with the actual protein amount per lane. Normalization with the so-called "loading control" was based on immunostaining of housekeeping proteins in the classical procedure, but is heading toward total protein staining recently, due to multiple benefits. At least seven different approaches for total protein staining have been described for western blot normalization: Ponceau S, stain-free techniques, Sypro Ruby, Epicocconone, Coomassie R-350, Amido Black, and Cy5. In order to avoid noise of signal, total protein staining should be performed before blocking of the membrane. Nevertheless, post-antibody stainings have been described as well.
Total protein staining allows the total protein that has been successfully transferred to the membrane to be visualised, allowing the user to check the uniformity of protein transfer and to perform subsequent normalization of the target protein with the actual protein amount per lane. Normalization with the so-called "loading control" was based on immunostaining of housekeeping proteins in the classical procedure, but is heading toward total protein staining recently, due to multiple benefits. At least seven different approaches for total protein staining have been described for western blot normalization: Ponceau S, stain-free techniques, Sypro Ruby, Epicocconone, Coomassie R-350, Amido Black, and Cy5. In order to avoid noise of signal, total protein staining should be performed before blocking of the membrane. Nevertheless, post-antibody stainings have been described as well.
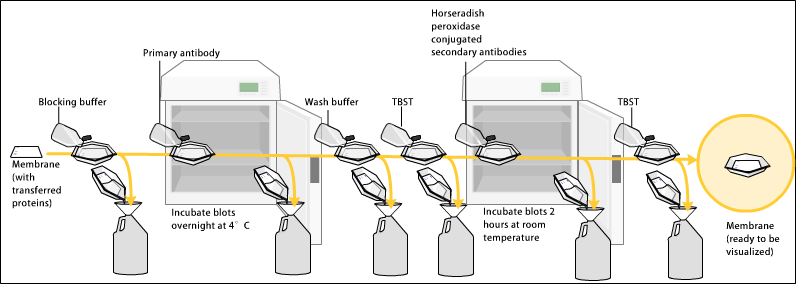
Blocking
Since the membrane has been chosen for its ability to bind protein and as both antibodies and the target are proteins, steps must be taken to prevent the interactions between the membrane and the antibody used for detection of the target protein. Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein – typically 3–5%bovine serum albumin
Bovine serum albumin (BSA or "Fraction V") is a serum albumin protein derived from cows. It is often used as a protein concentration standard in lab experiments.
The nickname "Fraction V" refers to albumin being the fifth fraction of the origi ...
(BSA) or non-fat dry milk (both are inexpensive) in tris-buffered saline (TBS) or I-Block, with a minute percentage (0.1%) of detergent such as Tween 20 or Triton X-100. Although non-fat dry milk is preferred due to its availability, an appropriate blocking solution is needed as not all proteins in milk are compatible with all the detection bands. The protein in the dilute solution attaches to the membrane in all places where the target proteins have not attached. Thus, when the antibody is added, it cannot bind to the membrane, and therefore the only available binding site is the specific target protein. This reduces background in the final product of the western blot, leading to clearer results, and eliminates false positives.
Incubation
 During the detection process, the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme; when exposed to an appropriate substrate, this enzyme drives a colorimetric reaction and produces a color. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
During the detection process, the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme; when exposed to an appropriate substrate, this enzyme drives a colorimetric reaction and produces a color. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
Primary antibody
The primary antibodies are generated when a host species or immune cell culture is exposed to the protein of interest (or a part thereof). Normally, this is part of the immune response, whereas here they are harvested and used as sensitive and specific detection tools that bind the protein directly. After blocking, a solution of primary antibody (generally between 0.5 and 5 micrograms/mL) diluted in either PBS or TBST wash buffer is incubated with the membrane under gentle agitation for typically an hour at room temperature, or overnight at 4°C. It can also be incubated at different temperatures, with lesser temperatures being associated with more binding, both specific (to the target protein, the "signal") and non-specific ("noise"). Following incubation, the membrane is washed several times in wash buffer to remove unbound primary antibody, and thereby minimize background. Typically, the wash buffer solution is composed of buffered saline solution with a small percentage of detergent, and sometimes with powdered milk or BSA.Secondary antibody
After rinsing the membrane to remove unbound primary antibody, the membrane is exposed to another antibody known as the secondary antibody. Antibodies come from animal sources (or animal sourced hybridoma cultures). The secondary antibody recognises and binds to the species-specific portion of the primary antibody. Therefore, an anti-mouse secondary antibody will bind to almost any mouse-sourced primary antibody, and can be referred to as an 'anti-species' antibody (e.g. anti-mouse, anti-goat etc.). To allow detection of the target protein, the secondary antibody is commonly linked to biotin or a reporterenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
such as alkaline phosphatase or horseradish peroxidase. This means that several secondary antibodies will bind to one primary antibody and enhance the signal, allowing the detection of proteins of a much lower concentration than would be visible by SDS-PAGE alone.
Horseradish peroxidase is commonly linked to secondary antibodies to allow the detection of the target protein by chemiluminescence. The chemiluminescent substrate is cleaved by horseradish peroxidase, resulting in the production of luminescence. Therefore, the production of luminescence is proportional to the amount of horseradish peroxidase-conjugated secondary antibody, and therefore, indirectly measures the presence of the target protein. A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot. A cheaper but less sensitive approach utilizes a 4-chloronaphthol stain with 1% hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3% ...
; the reaction of peroxide radicals with 4-chloronaphthol produces a dark purple stain that can be photographed without using specialized photographic film.
As with the ELISPOT and ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence ...
procedures, the enzyme can be provided with a substrate molecule that will be converted by the enzyme to a colored reaction product that will be visible on the membrane (see the figure below with blue bands).
Another method of secondary antibody detection utilizes a near-infrared fluorophore-linked antibody. The light produced from the excitation of a fluorescent dye is static, making fluorescent detection a more precise and accurate measure of the difference in the signal produced by labeled antibodies bound to proteins on a western blot. Proteins can be accurately quantified because the signal generated by the different amounts of proteins on the membranes is measured in a static state, as compared to chemiluminescence, in which light is measured in a dynamic state.
A third alternative is to use a radioactive label rather than an enzyme coupled to the secondary antibody, such as labeling an antibody-binding protein like ''Staphylococcus
''Staphylococcus'' is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical ( cocci), and form in grape-like clusters. ''Staphylococcus'' species are facultat ...
'' Protein A or Streptavidin with a radioactive isotope of iodine. Since other methods are safer, quicker, and cheaper, this method is now rarely used; however, an advantage of this approach is the sensitivity of auto-radiography-based imaging, which enables highly accurate protein quantification when combined with optical software (e.g. Optiquant).
One step
Historically, the probing process was performed in two steps because of the relative ease of producing primary and secondary antibodies in separate processes. This gives researchers and corporations huge advantages in terms of flexibility, reduction of cost, and adds an amplification step to the detection process. Given the advent of high-throughput protein analysis and lower limits of detection, however, there has been interest in developing one-step probing systems that would allow the process to occur faster and with fewer consumables. This requires a probe antibody which both recognizes the protein of interest and contains a detectable label, probes which are often available for known protein tags. The primary probe is incubated with the membrane in a manner similar to that for the primary antibody in a two-step process, and then is ready for direct detection after a series of wash steps.Detection and visualization
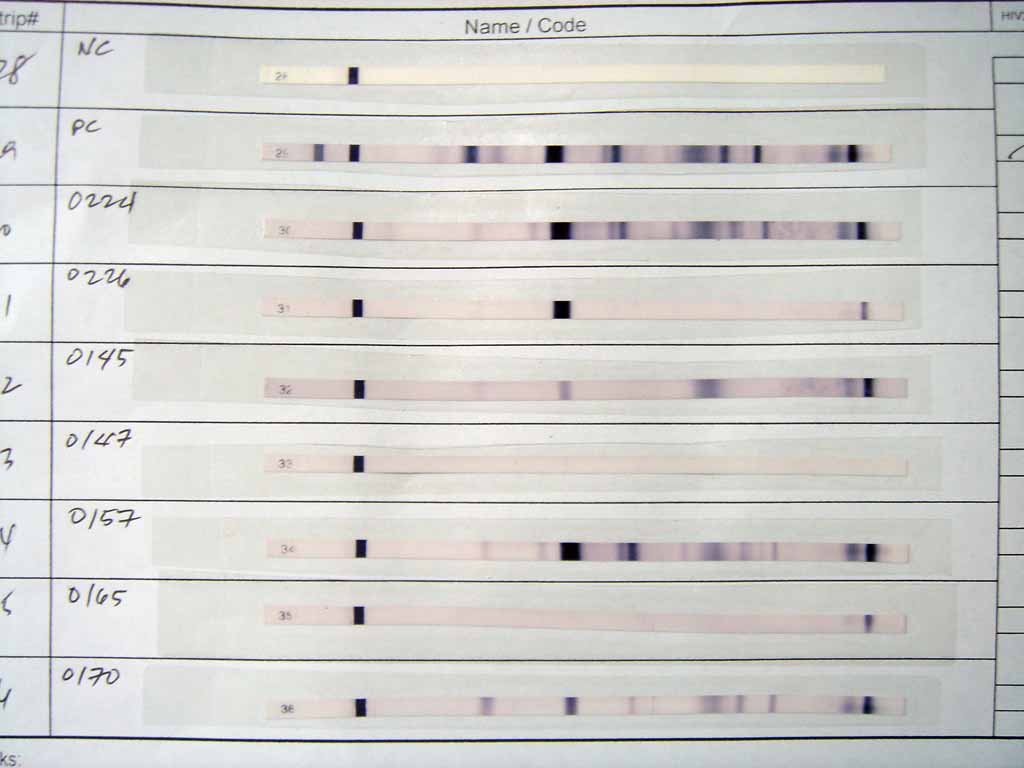
 After the unbound probes are washed away, the western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is commonly repeated for a structural protein, such as
After the unbound probes are washed away, the western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is commonly repeated for a structural protein, such as actin
Actin is a protein family, family of Globular protein, globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in myofibril, muscle fibrils. It is found in essentially all Eukaryote, eukaryotic cel ...
or tubulin, that should not change between samples. The amount of target protein is normalized to the structural protein to control between groups. A superior strategy is the normalization to the total protein visualized with trichloroethanol or epicocconone. This practice ensures correction for the amount of total protein on the membrane in case of errors or incomplete transfers. (see western blot normalization)
Colorimetric detection
The colorimetric detection method depends on incubation of the western blot with a substrate that reacts with the reporter enzyme (such as peroxidase) that is bound to the secondary antibody. This converts the soluble dye into an insoluble form of a different color that precipitates next to the enzyme and thereby stains the membrane. Development of the blot is then stopped by washing away the soluble dye. Protein levels are evaluated through densitometry (how intense the stain is) orspectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as sp ...
.
Chemiluminescent detection
Chemiluminescent detection methods depend on incubation of the western blot with a substrate that will luminesce when exposed to the reporter on the secondary antibody. The light is then detected by CCD cameras which capture a digital image of the western blot or photographic film. The use of film for western blot detection is slowly disappearing because of non linearity of the image (non accurate quantification). The image is analysed by densitometry, which evaluates the relative amount of protein staining and quantifies the results in terms of optical density. Newer software allows further data analysis such as molecular weight analysis if appropriate standards are used.Radioactive detection
Radioactive labels do not require enzyme substrates, but rather, allow the placement of medical X-ray film directly against the western blot, which develops as it is exposed to the label and creates dark regions which correspond to the protein bands of interest (see image above). The importance of radioactive detections methods is declining due to its hazardous radiation , because it is very expensive, health and safety risks are high, and ECL (enhanced chemiluminescence) provides a useful alternative.Fluorescent detection
 The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as a CCD camera equipped with appropriate emission filters which captures a digital image of the western blot and allows further data analysis such as molecular weight analysis and a quantitative western blot analysis. Fluorescence is considered to be one of the best methods for quantification but is less sensitive than chemiluminescence.
The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as a CCD camera equipped with appropriate emission filters which captures a digital image of the western blot and allows further data analysis such as molecular weight analysis and a quantitative western blot analysis. Fluorescence is considered to be one of the best methods for quantification but is less sensitive than chemiluminescence.
Secondary probing
One major difference between nitrocellulose and PVDF membranes relates to the ability of each to support "stripping" antibodies off and reusing the membrane for subsequent antibody probes. While there are well-established protocols available for stripping nitrocellulose membranes, the sturdier PVDF allows for easier stripping, and for more reuse before background noise limits experiments. Another difference is that, unlike nitrocellulose, PVDF must be soaked in 95% ethanol, isopropanol or methanol before use. PVDF membranes also tend to be thicker and more resistant to damage during use.2-D gel electrophoresis
Two-dimensional SDS-PAGE uses the principles and techniques outlined above. 2-D SDS-PAGE, as the name suggests, involves the migration of polypeptides in 2 dimensions. For example, in the first dimension, polypeptides are separated according to isoelectric point, while in the second dimension, polypeptides are separated according to theirmolecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
. The isoelectric point of a given protein is determined by the relative number of positively (e.g. lysine, arginine) and negatively (e.g. glutamate, aspartate) charged amino acids, with negatively charged amino acids contributing to a low isoelectric point and positively charged amino acids contributing to a high isoelectric point. Samples could also be separated first under nonreducing conditions using SDS-PAGE, and under reducing conditions in the second dimension, which breaks apart disulfide bonds that hold subunits together. SDS-PAGE might also be coupled with urea-PAGE for a 2-dimensional gel.
In principle, this method allows for the separation of all cellular proteins on a single large gel. A major advantage of this method is that it often distinguishes between different isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some iso ...
of a particular protein – e.g. a protein that has been phosphorylated (by addition of a negatively charged group). Proteins that have been separated can be cut out of the gel and then analysed by mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
, which identifies their molecular weight.
Presentation
Researchers use different software to process and align image-sections for elegant presentation of western blot results. Popular tools includSciugo
Microsoft PowerPoint
Microsoft PowerPoint is a presentation program, created by Robert Gaskins and Dennis Austin at a software company named Forethought, Inc. It was released on April 20, 1987, initially for Macintosh computers only. Microsoft acquired PowerPoi ...
, Adobe Illustrator and GIMP
GIMP ( ; GNU Image Manipulation Program) is a free and open-source raster graphics editor used for image manipulation (retouching) and image editing, free-form drawing, transcoding between different image file formats, and more specialized ...
.
See also
* Eastern blot * Far-eastern blot *Far-western blot
The far-western blot, or far-western blotting, is a molecular biological method based on the technique of western blot to detect protein-protein interaction ''in vitro''. Whereas western blot uses an antibody probe to detect a protein of interest, ...
* Fast parallel proteolysis
* Northwestern blot The northwestern blot, also known as the northwestern assay, is a hybrid analytical technique of the western blot and the northern blot, and is used in molecular biology to detect interactions between RNA and proteins. A related technique, the west ...
References
External links
* * Archived aGhostarchive
and th
Wayback Machine
* Archived a
Ghostarchive
and th
Wayback Machine
{{Molecular probes Diagnostic virology Protein methods Molecular biology Laboratory techniques Molecular biology techniques