|
Synthetic Antibody
Synthetic antibodies are affinity reagents generated entirely in vitro, thus completely eliminating animals from the production process. Synthetic antibodies include recombinant antibodies, nucleic acid aptamers and non-immunoglobulin protein scaffolds. As a consequence of their in vitro manufacturing method the antigen recognition site of synthetic antibodies can be engineered to any desired target and may extend beyond the typical immune repertoire offered by natural antibodies. Synthetic antibodies are being developed for use in research, diagnostic and therapeutic applications. Synthetic antibodies can be used in all applications where traditional monoclonal or polyclonal antibodies are used and offer many inherent advantages over animal-derived antibodies, including comparatively low production costs, reagent reproducibility and increased affinity, specificity and stability across a range of experimental conditions. Recombinant antibodies Recombinant antibodies are monoclon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant Antibodies
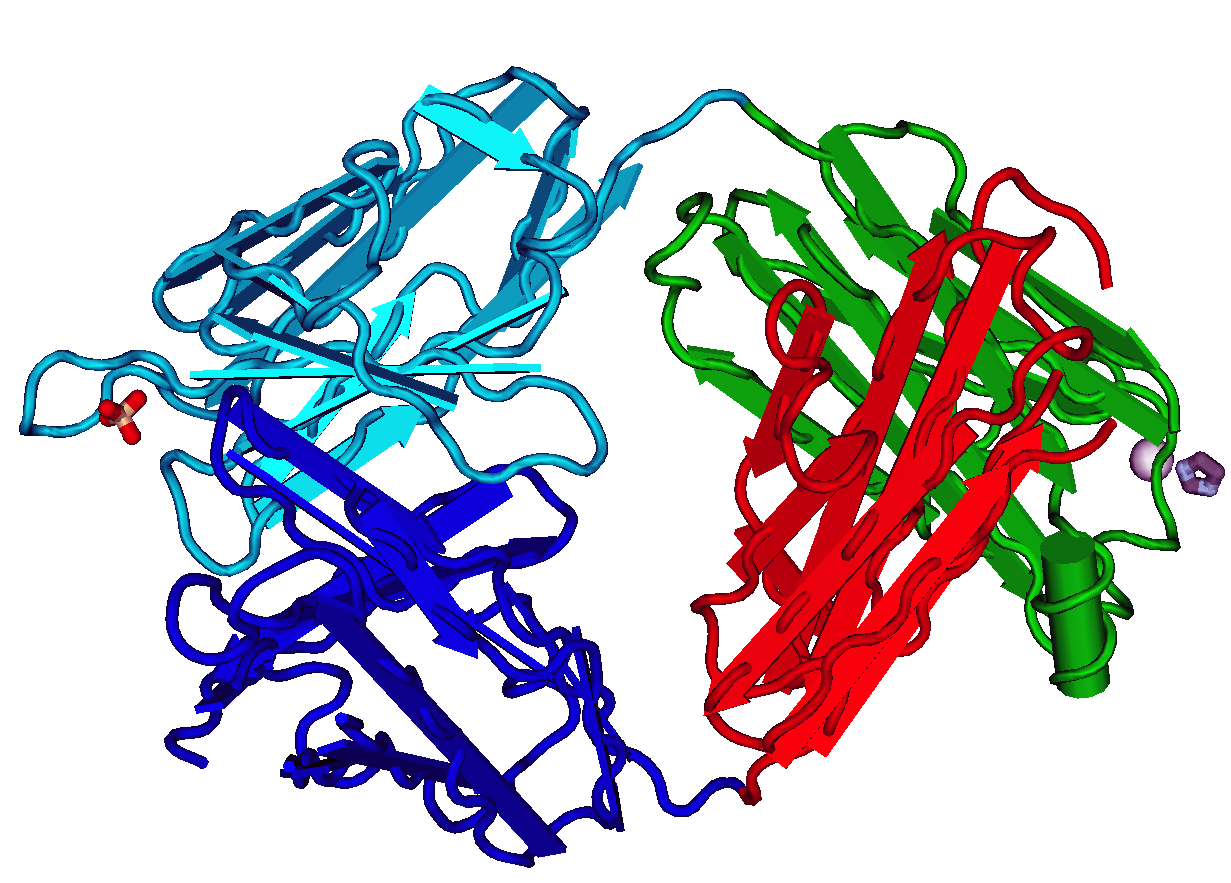
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity. Structure and characterization Formats There are several known formats of recombinant antibodies which are commonly produced. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. The term ''antigen'' originally referred to a substance that is an antibody generator. Antigens can be proteins, peptides (amino acid chains), polysaccharides (chains of monosaccharides/simple sugars), lipids, or nucleic acids. Antigens are recognized by antigen receptors, including antibodies and T-cell receptors. Diverse antigen receptors are made by cells of the immune system so that each cell has a specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize that antigen are activated and expanded, a process known as clonal selection. In most cases, an antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cross-react and bind more than one antigen. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune Repertoire
The immune repertoire encompasses the different sub-types an organism's immune system makes of immunoglobulins or T-cell receptors. These help recognise pathogens in most vertebrates. The sub-types, all differing slightly from each other, can amount to tens of thousands, or millions in a given organism. Such a wide variety increases the odds of having a sub-type that recognises one of the many pathogens an organism may encounter. Too few sub-types and the pathogen can avoid the immune system, unchallenged, leading to disease. Development Lymphocytes generate the immune repertoire by recombining the genes encoding immunoglobulins and T cell receptors through V(D)J recombination. Although there are only a few of these genes, all their possible combinations can result in a wide variety of immune repertoire proteins. Through selection, cells with autoreactive proteins (and thus may cause autoimmunity) are removed, while cells that may actually detect an invading organism are kept. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyclonal Antibodies
Polyclonal antibodies (pAbs) are antibodies that are secreted by different B cell lineages within the body (whereas monoclonal antibodies come from a single cell lineage). They are a collection of immunoglobulin molecules that react against a specific antigen, each identifying a different epitope. Production The general procedure to produce polyclonal antibodies is as follows: # Antigen preparation # Adjuvant selection and preparation # Animal selection # Injection process # Blood serum extraction An antigen/adjuvant conjugate is injected into an animal of choice to initiate an amplified immune response. After a series of injections over a specific length of time, the animal is expected to have created antibodies against the conjugate. Blood is then extracted from the animal and then purified to obtain the antibody of interest. Inoculation is performed on a suitable mammal, such as a mouse, rabbit or goat. Larger mammals are often preferred as the amount of serum that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity, binding only to the same epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody-secreting plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one monoclonal antibody to two epitopes. It is possible to produce monoclonal antibodies that specifically bind to virtually any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibodies are being used on a clinical level for both the diagnosis and therapy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner at neut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ScFv
A single-chain variable fragment (scFv) is not actually a fragment of an antibody, but instead is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, connected with a short linker peptide of ten to about 25 amino acids. The linker is usually rich in glycine for flexibility, as well as serine or threonine for solubility, and can either connect the N-terminus of the VH with the C-terminus of the VL, or ''vice versa''. This protein retains the specificity of the original immunoglobulin, despite removal of the constant regions and the introduction of the linker. The image to the right shows how this modification usually leaves the specificity unaltered. These molecules were created to facilitate phage display, where it is highly convenient to express the antigen-binding domain as a single peptide. As an alternative, scFv can be created directly from subcloned heavy and light chains derived from a hybridoma. ScFvs have many uses, e. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
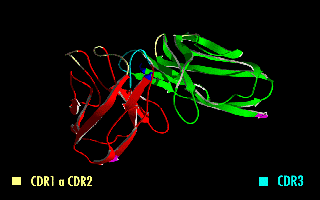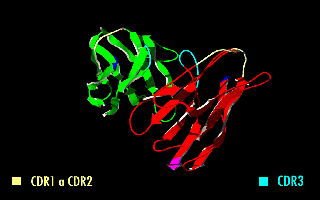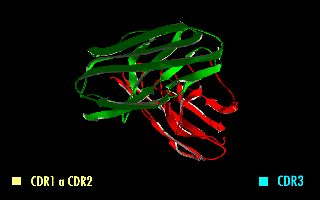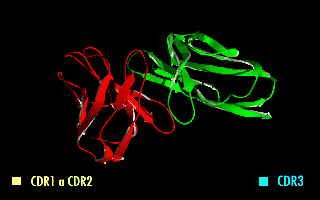
Complementarity-determining Region
Complementarity-determining regions (CDRs) are part of the variable chains in immunoglobulins (antibodies) and T cell receptors, generated by B-cells and T-cells respectively, where these molecules bind to their specific antigen. A set of CDRs constitutes a paratope. As the most variable parts of the molecules, CDRs are crucial to the diversity of antigen specificities generated by lymphocytes. Location and structure There are three CDRs (CDR1, CDR2 and CDR3), arranged non-consecutively, on the amino acid sequence of a variable domain of an antigen receptor. Since the antigen receptors are typically composed of two variable domains (on two different polypeptide chains, heavy and light chain), there are six CDRs for each antigen receptor that can collectively come into contact with the antigen. A single antibody molecule has two antigen receptors and therefore contains twelve CDRs total. There are three CDR loops per variable domain in antibodies. Sixty CDRs can be found on a pen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acids
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is the ribose derivative deoxyribose, the polymer is DNA. Nucleic acids are naturally occurring chemical compounds that serve as the primary information-carrying molecules in cells and make up the genetic material. Nucleic acids are found in abundance in all living things, where they create, encode, and then store information of every living cell of every life-form on Earth. In turn, they function to transmit and express that information inside and outside the cell nucleus to the interior operations of the cell and ultimately to the next generation of each living organism. The encoded information is co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aptamer
Aptamers are short sequences of artificial DNA, RNA, XNA, or peptide that bind a specific target molecule, or family of target molecules. They exhibit a range of affinities ( KD in the pM to μM range), with little or no off-target binding and are sometimes classified as chemical antibodies. Aptamers and antibodies can be used in many of the same applications, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes (see antibody replacement). Aptamers are used in biological lab research and medical tests. If multiple aptamers are combined into a single assay, they can measure large numbers of different proteins in a sample. They can be used to identify molecular markers of disease, or can function as drugs, drug delivery systems and controlled drug release systems. They a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aptamer
Aptamers are short sequences of artificial DNA, RNA, XNA, or peptide that bind a specific target molecule, or family of target molecules. They exhibit a range of affinities ( KD in the pM to μM range), with little or no off-target binding and are sometimes classified as chemical antibodies. Aptamers and antibodies can be used in many of the same applications, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes (see antibody replacement). Aptamers are used in biological lab research and medical tests. If multiple aptamers are combined into a single assay, they can measure large numbers of different proteins in a sample. They can be used to identify molecular markers of disease, or can function as drugs, drug delivery systems and controlled drug release systems. They a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affimer
Affimer molecules are small proteins that bind to target proteins with affinity in the nanomolar range. These engineered non-antibody binding proteins are designed to mimic the molecular recognition characteristics of monoclonal antibodies in different applications. These affinity reagents have been optimized to increase their stability, make them tolerant to a range of temperatures and pH, reduce their size, and to increase their expression in ''E.coli'' and mammalian cells. Development Affimer proteins were developed initially at the MRC Cancer Cell Unit in Cambridge then across two laboratories at the University of Leeds. Derived from the cysteine protease inhibitor family of cystatins, which function in nature as cysteine protease inhibitors, these 12–14 kDa proteins share the common tertiary structure of an alpha-helix lying on top of an anti-parallel beta-sheet. Affimer proteins display two peptide loops that can all be randomized to bind to desired target proteins, in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |