Uranium Mines In The United States on:
[Wikipedia]
[Google]
[Amazon]
Uranium is a chemical element with the
 When refined, uranium is a silvery white, weakly radioactive metal. It has a Mohs hardness of 6, sufficient to scratch glass and approximately equal to that of titanium, rhodium, manganese and
When refined, uranium is a silvery white, weakly radioactive metal. It has a Mohs hardness of 6, sufficient to scratch glass and approximately equal to that of titanium, rhodium, manganese and
 The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of
The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of
 The main use of uranium in the civilian sector is to fuel
The main use of uranium in the civilian sector is to fuel  Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass and in
Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass and in 
 Uranium was also used in photographic chemicals (especially
Uranium was also used in photographic chemicals (especially
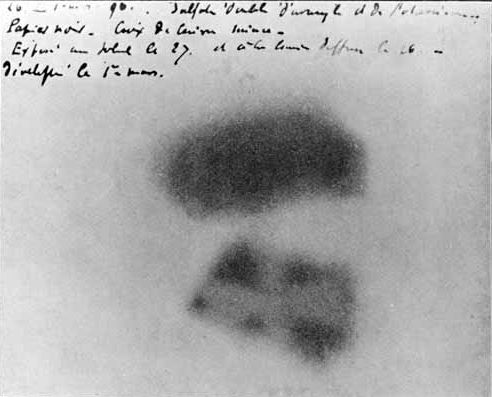 The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with
The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with
 A team led by
A team led by
 Two major types of atomic bombs were developed by the United States during World War II: a uranium-based device (codenamed " Little Boy") whose fissile material was highly enriched uranium, and a plutonium-based device (see Trinity test and " Fat Man") whose plutonium was derived from uranium-238. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the
Two major types of atomic bombs were developed by the United States during World War II: a uranium-based device (codenamed " Little Boy") whose fissile material was highly enriched uranium, and a plutonium-based device (see Trinity test and " Fat Man") whose plutonium was derived from uranium-238. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the
 The X-10 Graphite Reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, formerly known as the Clinton Pile and X-10 Pile, was the world's second artificial nuclear reactor (after Enrico Fermi's Chicago Pile) and was the first reactor designed and built for continuous operation.
The X-10 Graphite Reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, formerly known as the Clinton Pile and X-10 Pile, was the world's second artificial nuclear reactor (after Enrico Fermi's Chicago Pile) and was the first reactor designed and built for continuous operation.
 Above-ground nuclear tests by the Soviet Union and the United States in the 1950s and early 1960s and by France into the 1970s and 1980s spread a significant amount of fallout from uranium daughter isotopes around the world. Additional fallout and pollution occurred from several nuclear accidents.
Uranium miners have a higher incidence of cancer. An excess risk of lung cancer among
Above-ground nuclear tests by the Soviet Union and the United States in the 1950s and early 1960s and by France into the 1970s and 1980s spread a significant amount of fallout from uranium daughter isotopes around the world. Additional fallout and pollution occurred from several nuclear accidents.
Uranium miners have a higher incidence of cancer. An excess risk of lung cancer among

 Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is the 51st element in order of abundance in the Earth's crust. Uranium is also the highest-numbered element to be found naturally in significant quantities on Earth and is almost always found combined with other elements. The decay of uranium, thorium, and potassium-40 in the Earth's
Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is the 51st element in order of abundance in the Earth's crust. Uranium is also the highest-numbered element to be found naturally in significant quantities on Earth and is almost always found combined with other elements. The decay of uranium, thorium, and potassium-40 in the Earth's  Some bacteria, such as '' Shewanella putrefaciens'', '' Geobacter metallireducens'' and some strains of ''
Some bacteria, such as '' Shewanella putrefaciens'', '' Geobacter metallireducens'' and some strains of ''
 Worldwide production of U3O8 (yellowcake) in 2013 amounted to 70,015 tonnes, of which 22,451 t (32%) was mined in Kazakhstan. Other important uranium mining countries are Canada (9,331 t),
Worldwide production of U3O8 (yellowcake) in 2013 amounted to 70,015 tonnes, of which 22,451 t (32%) was mined in Kazakhstan. Other important uranium mining countries are Canada (9,331 t),
File:U production-demand.png, World uranium production (mines) and demand
File:Yellowcake.jpg, alt=A yellow sand-like rhombic mass on black background., Yellowcake is a concentrated mixture of uranium oxides that is further refined to extract pure uranium.
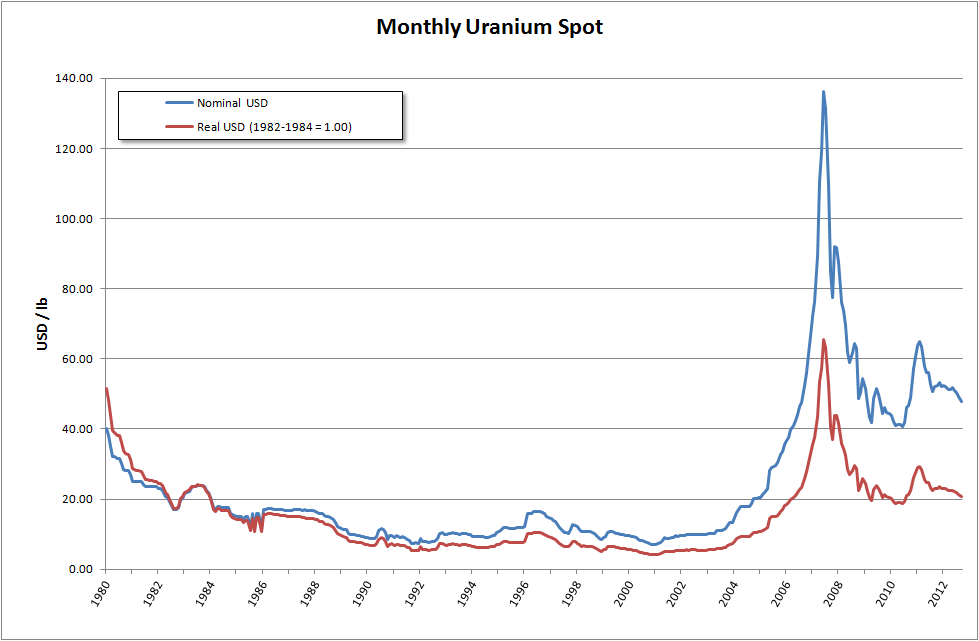
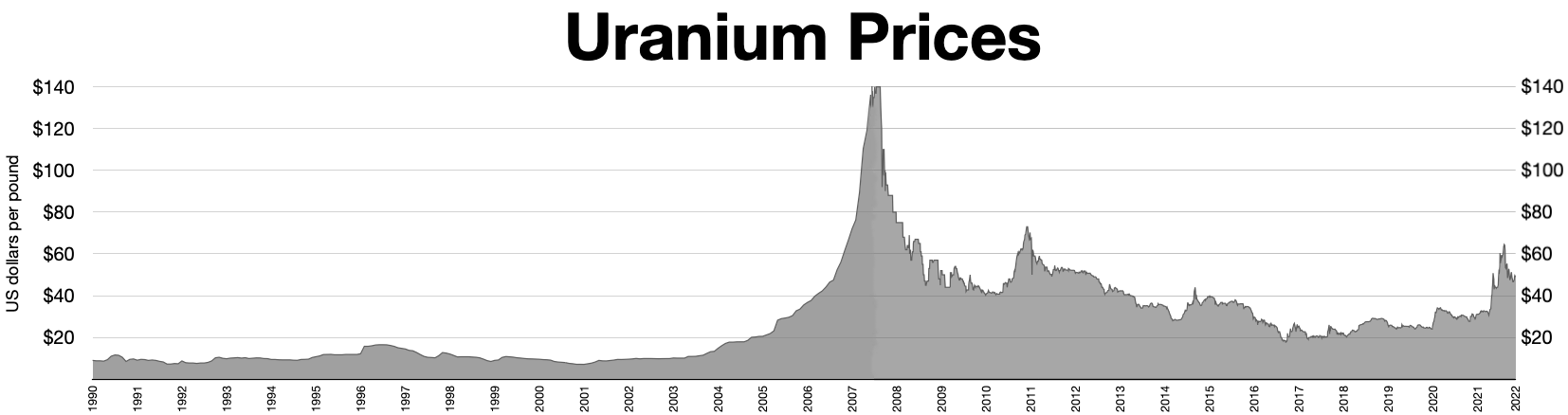 It is estimated that 5.5 million tonnes of uranium exists in ore reserves that are economically viable at US$59 per lb of uranium, while 35 million tonnes are classed as mineral resources (reasonable prospects for eventual economic extraction). Prices went from about $10/lb in May 2003 to $138/lb in July 2007. This has caused a big increase in spending on exploration, with US$200 million being spent worldwide in 2005, a 54% increase on the previous year. This trend continued through 2006, when expenditure on exploration rocketed to over $774 million, an increase of over 250% compared to 2004. The OECD Nuclear Energy Agency said exploration figures for 2007 would likely match those for 2006.
Australia has 31% of the world's known uranium ore reserves and the world's largest single uranium deposit, located at the Olympic Dam Mine in South Australia. There is a significant reserve of uranium in Bakouma, a
It is estimated that 5.5 million tonnes of uranium exists in ore reserves that are economically viable at US$59 per lb of uranium, while 35 million tonnes are classed as mineral resources (reasonable prospects for eventual economic extraction). Prices went from about $10/lb in May 2003 to $138/lb in July 2007. This has caused a big increase in spending on exploration, with US$200 million being spent worldwide in 2005, a 54% increase on the previous year. This trend continued through 2006, when expenditure on exploration rocketed to over $774 million, an increase of over 250% compared to 2004. The OECD Nuclear Energy Agency said exploration figures for 2007 would likely match those for 2006.
Australia has 31% of the world's known uranium ore reserves and the world's largest single uranium deposit, located at the Olympic Dam Mine in South Australia. There is a significant reserve of uranium in Bakouma, a
 In 2005, seventeen countries produced concentrated uranium oxides: Canada (27.9% of world production),
In 2005, seventeen countries produced concentrated uranium oxides: Canada (27.9% of world production),

 Salts of many oxidation states of uranium are water- soluble and may be studied in
Salts of many oxidation states of uranium are water- soluble and may be studied in
 All uranium fluorides are created using uranium tetrafluoride (); itself is prepared by hydrofluorination of uranium dioxide. Reduction of with hydrogen at 1000 °C produces uranium trifluoride (). Under the right conditions of temperature and pressure, the reaction of solid with gaseous uranium hexafluoride () can form the intermediate fluorides of , , and .
At room temperatures, has a high vapor pressure, making it useful in the gaseous diffusion process to separate the rare uranium-235 from the common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:
: + 4 HF → + 2 (500 °C, endothermic)
: + → (350 °C, endothermic)
The resulting , a white solid, is highly reactive (by fluorination), easily
All uranium fluorides are created using uranium tetrafluoride (); itself is prepared by hydrofluorination of uranium dioxide. Reduction of with hydrogen at 1000 °C produces uranium trifluoride (). Under the right conditions of temperature and pressure, the reaction of solid with gaseous uranium hexafluoride () can form the intermediate fluorides of , , and .
At room temperatures, has a high vapor pressure, making it useful in the gaseous diffusion process to separate the rare uranium-235 from the common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:
: + 4 HF → + 2 (500 °C, endothermic)
: + → (350 °C, endothermic)
The resulting , a white solid, is highly reactive (by fluorination), easily
 In nature, uranium is found as uranium-238 (99.2742%) and uranium-235 (0.7204%). Isotope separation concentrates (enriches) the fissionable uranium-235 for nuclear weapons and most nuclear power plants, except for gas cooled reactors and pressurised heavy water reactors. Most neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the
In nature, uranium is found as uranium-238 (99.2742%) and uranium-235 (0.7204%). Isotope separation concentrates (enriches) the fissionable uranium-235 for nuclear weapons and most nuclear power plants, except for gas cooled reactors and pressurised heavy water reactors. Most neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the
U.S. EPA: Radiation Information for Uranium
from
Nuclear fuel data and analysis
from the
Current market price of uranium
Annotated bibliography for uranium from the Alsos Digital Library
NLM Hazardous Substances Databank—Uranium, Radioactive
Mining Uranium at Namibia's Langer Heinrich Mine
World Nuclear News
ATSDR Case Studies in Environmental Medicine: Uranium Toxicity
U.S. Department of Health and Human Services
Uranium
at '' The Periodic Table of Videos'' (University of Nottingham) {{Authority control Chemical elements Actinides Nuclear fuels Nuclear materials Suspected male-mediated teratogens Manhattan Project
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. A uranium atom has 92 proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes fr ...
are uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
(which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted
''Extracted'', also known as ''Extraction'' in the UK, is an independent 2012 American science fiction thriller directed and written by Nir Paniry. Sasha Roiz stars as a scientist whose consciousness becomes trapped in the mind of a convict (Dom ...
from uranium-bearing minerals such as uraninite.
In nature, uranium is found as uranium-238 (99.2739–99.2752%), uranium-235 (0.7198–0.7202%), and a very small amount of uranium-234 (0.0050–0.0059%). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million
One million (1,000,000), or one thousand thousand, is the natural number following 999,999 and preceding 1,000,001. The word is derived from the early Italian ''millione'' (''milione'' in modern Italian), from ''mille'', "thousand", plus the au ...
years, making them useful in dating the age of the Earth
The age of Earth is estimated to be 4.54 ± 0.05 billion years This age may represent the age of Earth's accretion, or core formation, or of the material from which Earth formed. This dating is based on evidence from radiometric age-dating of ...
.
Many contemporary uses of uranium exploit its unique nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
*Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
*Nuclear space
* Nuclear ...
properties. Uranium-235 is the only naturally occurring fissile isotope, which makes it widely used in nuclear power plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a electric generator, generato ...
s and nuclear weapons. However, because of the tiny amounts found in nature, uranium needs to undergo enrichment
Enrichment may refer to:
* Behavioral enrichment, the practice of providing animals under managed care with stimuli such as natural and artificial objects
* Data enrichment, appending or enhancing data with relevant context from other sources, se ...
so that enough uranium-235 is present. Uranium-238 is fissionable by fast neutrons, and is fertile
Fertility is the capability to produce offspring through reproduction following the onset of sexual maturity. The fertility rate is the average number of children born by a female during her lifetime and is quantified demographically. Fertilit ...
, meaning it can be transmuted to fissile plutonium-239 in a nuclear reactor. Another fissile isotope, uranium-233, can be produced from natural thorium and is studied for future industrial use in nuclear technology. Uranium-238 has a small probability for spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdo ...
or even induced fission with fast neutrons; uranium-235 and to a lesser degree uranium-233 have a much higher fission cross-section for slow neutrons. In sufficient concentration, these isotopes maintain a sustained nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
. This generates the heat in nuclear power reactors, and produces the fissile material for nuclear weapons. Depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, hav ...
(238U) is used in kinetic energy penetrator
A kinetic energy penetrator (KEP), also known as long-rod penetrator (LRP), is a type of ammunition designed to penetrate vehicle armour using a flechette-like, high-sectional density projectile. Like a bullet or kinetic energy weapon, this type ...
s and armor plating.. Uranium is used as a colorant in uranium glass, producing lemon yellow to green colors. Uranium glass fluoresces green in ultraviolet light. It was also used for tinting and shading in early photography.
The 1789 discovery of uranium in the mineral pitchblende is credited to Martin Heinrich Klaproth, who named the new element after the recently discovered planet Uranus. Eugène-Melchior Péligot was the first person to isolate the metal and its radioactive properties were discovered in 1896 by Henri Becquerel. Research by Otto Hahn, Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on rad ...
, Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" and ...
and others, such as J. Robert Oppenheimer starting in 1934 led to its use as a fuel in the nuclear power industry and in '' Little Boy'', the first nuclear weapon used in war. An ensuing arms race
An arms race occurs when two or more groups compete in military superiority. It consists of a competition between two or more states to have superior armed forces; a competition concerning production of weapons, the growth of a military, and t ...
during the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because the ...
between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used uranium metal and uranium-derived plutonium-239. The security of those weapons is closely monitored. Since around 2000, plutonium obtained by dismantling Cold War-era bombs is used as fuel for nuclear reactors. The development and deployment of these nuclear reactors continue on a global base as they are powerful sources of CO2-free energy.
Characteristics
niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
. It is malleable, ductile, slightly paramagnetic, strongly electropositive and a poor electrical conductor. Uranium metal has a very high density of 19.1 g/cm3, denser than lead (11.3 g/cm3), but slightly less dense than tungsten and gold (19.3 g/cm3).
Uranium metal reacts with almost all non-metal elements (with the exception of the noble gases) and their compounds, with reactivity increasing with temperature. Hydrochloric and nitric acids dissolve uranium, but non-oxidizing acids other than hydrochloric acid attack the element very slowly. When finely divided, it can react with cold water; in air, uranium metal becomes coated with a dark layer of uranium oxide. Uranium in ores is extracted chemically and converted into uranium dioxide or other chemical forms usable in industry.
Uranium-235 was the first isotope that was found to be fissile. Other naturally occurring isotopes are fissionable, but not fissile. On bombardment with slow neutrons, its uranium-235 isotope will most of the time divide into two smaller nuclei, releasing nuclear binding energy and more neutrons. If too many of these neutrons are absorbed by other uranium-235 nuclei, a nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
occurs that results in a burst of heat or (in special circumstances) an explosion. In a nuclear reactor, such a chain reaction is slowed and controlled by a neutron poison, absorbing some of the free neutrons. Such neutron absorbent materials are often part of reactor control rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing ...
s (see nuclear reactor physics for a description of this process of reactor control).
As little as of uranium-235 can be used to make an atomic bomb. The nuclear weapon detonated over Hiroshima
is the capital of Hiroshima Prefecture in Japan. , the city had an estimated population of 1,199,391. The gross domestic product (GDP) in Greater Hiroshima, Hiroshima Urban Employment Area, was US$61.3 billion as of 2010. Kazumi Matsui h ...
, called '' Little Boy'', relied on uranium fission. However, the first nuclear bomb (the ''Gadget'' used at Trinity) and the bomb that was detonated over Nagasaki ('' Fat Man'') were both plutonium bombs.
Uranium metal has three allotropic forms:
* α (orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
) stable up to . Orthorhombic, space group No. 63, ''Cmcm'', lattice parameters ''a''= 285.4 pm, ''b'' = 587 pm, ''c'' = 495.5 pm.
* β ( tetragonal) stable from . Tetragonal, space group ''P''42/''mnm'', ''P''42''nm'', or ''P''4''n''2, lattice parameters ''a'' = 565.6 pm, ''b'' = ''c'' = 1075.9 pm.
* γ ( body-centered cubic) from to melting point—this is the most malleable and ductile state. Body-centered cubic, lattice parameter ''a'' = 352.4 pm.
Applications
Military
 The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of
The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, hav ...
(DU) alloyed with 1–2% other elements, such as titanium or molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
. At high impact speed, the density, hardness, and pyrophoricity of the projectile enable the destruction of heavily armored targets. Tank armor and other removable vehicle armor can also be hardened with depleted uranium plates. The use of depleted uranium became politically and environmentally contentious after the use of such munitions by the US, UK and other countries during wars in the Persian Gulf and the Balkans raised questions concerning uranium compounds left in the soil (see Gulf War syndrome).
Depleted uranium is also used as a shielding material in some containers used to store and transport radioactive materials. While the metal itself is radioactive, its high density makes it more effective than lead in halting radiation from strong sources such as radium. Other uses of depleted uranium include counterweights for aircraft control surfaces, as ballast for missile re-entry vehicles and as a shielding material. Due to its high density, this material is found in inertial guidance system
An inertial navigation system (INS) is a navigation device that uses motion sensors (accelerometers), rotation sensors ( gyroscopes) and a computer to continuously calculate by dead reckoning the position, the orientation, and the velocity (dire ...
s and in gyroscopic compasses. Depleted uranium is preferred over similarly dense metals due to its ability to be easily machined and cast as well as its relatively low cost.. The main risk of exposure to depleted uranium is chemical poisoning by uranium oxide rather than radioactivity (uranium being only a weak alpha emitter).
During the later stages of World War II, the entire Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because the ...
, and to a lesser extent afterwards, uranium-235 has been used as the fissile explosive material to produce nuclear weapons. Initially, two major types of fission bombs were built: a relatively simple device that uses uranium-235 and a more complicated mechanism that uses plutonium-239 derived from uranium-238. Later, a much more complicated and far more powerful type of fission/fusion bomb ( thermonuclear weapon) was built, that uses a plutonium-based device to cause a mixture of tritium and deuterium to undergo nuclear fusion. Such bombs are jacketed in a non-fissile (unenriched) uranium case, and they derive more than half their power from the fission of this material by fast neutrons from the nuclear fusion process.
Civilian
 The main use of uranium in the civilian sector is to fuel
The main use of uranium in the civilian sector is to fuel nuclear power plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a electric generator, generato ...
s. One kilogram of uranium-235 can theoretically produce about 20 terajoules
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applied. ...
of energy (2 joules), assuming complete fission; as much energy as 1.5 million kilograms (1,500 tonnes) of coal.
Commercial nuclear power plants use fuel that is typically enriched to around 3% uranium-235. The CANDU
The CANDU (Canada Deuterium Uranium) is a Canadian pressurized heavy-water reactor design used to generate electric power. The acronym refers to its deuterium oxide ( heavy water) moderator and its use of (originally, natural) uranium fuel. C ...
and Magnox designs are the only commercial reactors capable of using unenriched uranium fuel. Fuel used for United States Navy reactors is typically highly enriched in uranium-235 (the exact values are classified
Classified may refer to:
General
*Classified information, material that a government body deems to be sensitive
*Classified advertising or "classifieds"
Music
*Classified (rapper) (born 1977), Canadian rapper
*The Classified, a 1980s American roc ...
). In a breeder reactor, uranium-238 can also be converted into plutonium through the following reaction:
: + n +
 Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass and in
Before (and, occasionally, after) the discovery of radioactivity, uranium was primarily used in small amounts for yellow glass and pottery glazes, such as uranium glass and in Fiestaware
Fiesta is a line of ceramic glazed dinnerware manufactured and marketed by the Fiesta Tableware Company of Newell, West Virginia since its introduction in 1936, with a hiatus from 1973 to 1985. Fiesta is noted for its Art Deco styling and its ra ...
.
The discovery and isolation of radium in uranium ore (pitchblende) by Marie Curie sparked the development of uranium mining to extract the radium, which was used to make glow-in-the-dark paints for clock and aircraft dials. This left a prodigious quantity of uranium as a waste product, since it takes three tonnes of uranium to extract one gram of radium. This waste product was diverted to the glazing industry, making uranium glazes very inexpensive and abundant. Besides the pottery glazes, uranium tile
Uranium tiles have been used in the ceramics industry for many centuries, as uranium oxide makes an excellent ceramic glaze, and is reasonably abundant. In addition to its medical usage, radium was used in the 1920s and 1930s for making watch, ...
glazes accounted for the bulk of the use, including common bathroom and kitchen tiles which can be produced in green, yellow, mauve, black, blue, red and other colors.

 Uranium was also used in photographic chemicals (especially
Uranium was also used in photographic chemicals (especially uranium nitrate
Uranyl nitrate is a water-soluble yellow uranium salt with the formula . The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels.
Uranyl nitrate can be prepa ...
as a toner), in lamp filaments for stage lighting
Stage lighting is the craft of lighting as it applies to the production of theater, dance, opera, and other performance arts.
bulbs, to improve the appearance of dentures, and in the leather and wood industries for stains and dyes. Uranium salts are mordants of silk or wool. Uranyl acetate and uranyl formate are used as electron-dense "stains" in transmission electron microscopy, to increase the contrast of biological specimens in ultrathin sections and in negative staining of viruses, isolated cell organelles and macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
s.
The discovery of the radioactivity of uranium ushered in additional scientific and practical uses of the element. The long half-life of the isotope uranium-238 (4.47 years) makes it well-suited for use in estimating the age of the earliest igneous rocks and for other types of radiometric dating, including uranium–thorium dating, uranium–lead dating and uranium–uranium dating. Uranium metal is used for X-ray targets in the making of high-energy X-rays.
History
Pre-discovery use
The use of uranium in its naturaloxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
form dates back to at least the year 79 CE, when it was used in the Roman Empire to add a yellow color to ceramic glazes. Yellow glass with 1% uranium oxide was found in a Roman villa on Cape Posillipo in the Bay of Naples
A bay is a recessed, coastal body of water that directly connects to a larger main body of water, such as an ocean, a lake, or another bay. A large bay is usually called a gulf, sea, sound, or bight. A cove is a small, circular bay with a narr ...
, Italy, by R. T. Gunther of the University of Oxford in 1912.. Starting in the late Middle Ages, pitchblende was extracted from the Habsburg
The House of Habsburg (), alternatively spelled Hapsburg in Englishgerman: Haus Habsburg, ; es, Casa de Habsburgo; hu, Habsburg család, it, Casa di Asburgo, nl, Huis van Habsburg, pl, dom Habsburgów, pt, Casa de Habsburgo, la, Domus Hab ...
silver mines in Joachimsthal Joachimsthal, sometimes spelled Joachimstal, may refer to:
Places
* Joachimsthal, Bohemia, former name of Jáchymov,, Czechia, famous for its silver and uranium mines and which gave its name to the ''Joachimsthaler'' currency
* Joachimsthal, Bra ...
, Bohemia
Bohemia ( ; cs, Čechy ; ; hsb, Čěska; szl, Czechy) is the westernmost and largest historical region of the Czech Republic. Bohemia can also refer to a wider area consisting of the historical Lands of the Bohemian Crown ruled by the Bohem ...
(now Jáchymov in the Czech Republic), and was used as a coloring agent in the local glassmaking industry. In the early 19th century, the world's only known sources of uranium ore were these mines. Mining for uranium in the Ore Mountains ceased on the German side after the Cold War ended and SDAG Wismut was wound down. On the Czech side there were attempts during the uranium price bubble of 2007 to restart mining, but those were quickly abandoned following a fall in uranium prices.
Discovery
 The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with
The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
.. Klaproth assumed the yellow substance was the oxide of a yet-undiscovered element and heated it with charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, cal ...
to obtain a black powder, which he thought was the newly discovered metal itself (in fact, that powder was an oxide of uranium). He named the newly discovered element after the planet Uranus (named after the primordial Greek god of the sky), which had been discovered eight years earlier by William Herschel.
In 1841, Eugène-Melchior Péligot, Professor of Analytical Chemistry at the Conservatoire National des Arts et Métiers (Central School of Arts and Manufactures) in Paris, isolated the first sample of uranium metal by heating uranium tetrachloride with potassium.
Henri Becquerel discovered radioactivity by using uranium in 1896. Becquerel made the discovery in Paris by leaving a sample of a uranium salt, K2UO2(SO4)2 (potassium uranyl sulfate), on top of an unexposed photographic plate
Photographic plates preceded photographic film as a capture medium in photography, and were still used in some communities up until the late 20th century. The light-sensitive emulsion of silver salts was coated on a glass plate, typically thinn ...
in a drawer and noting that the plate had become "fogged". He determined that a form of invisible light or rays emitted by uranium had exposed the plate.
During World War I when the Central Powers suffered a shortage of molybdenum to make artillery gun barrels and high speed tool steels they routinely substituted ferrouranium alloys which present many of the same physical characteristics. When this practice became known in 1916 the USA government requested several prominent universities to research these uses for uranium and tools made with these formulas remained in use for several decades only ending when the Manhattan Project and the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because the ...
placed a large demand on uranium for fission research and weapon development.
Fission research
 A team led by
A team led by Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" and ...
in 1934 observed that bombarding uranium with neutrons produces the emission of beta rays
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
( electrons or positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collides ...
s from the elements produced; see beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
). The fission products were at first mistaken for new elements with atomic numbers 93 and 94, which the Dean of the Faculty of Rome, Orso Mario Corbino, christened ''ausonium
Ausenium (atomic symbol Ao) was the name assigned to the element with atomic number 93, now known as neptunium. It was named after a Greek name of Italy, Ausonia (disambiguation), Ausonia.
The same team assigned the name hesperium to element 94, ...
'' and '' hesperium'', respectively. The experiments leading to the discovery of uranium's ability to fission (break apart) into lighter elements and release binding energy were conducted by Otto Hahn and Fritz Strassmann. in Hahn's laboratory in Berlin. Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on rad ...
and her nephew, the physicist Otto Robert Frisch, published the physical explanation in February 1939 and named the process "nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
". Soon after, Fermi hypothesized that the fission of uranium might release enough neutrons to sustain a fission reaction. Confirmation of this hypothesis came in 1939, and later work found that on average about 2.5 neutrons are released by each fission of the rare uranium isotope uranium-235. Fermi urged Alfred O. C. Nier
Alfred Otto Carl Nier (May 28, 1911 – May 16, 1994) was an American physicist who pioneered the development of mass spectrometry. He was the first to use mass spectrometry to isolate uranium-235 which was used to demonstrate that 235U could unde ...
to separate uranium isotopes for determination of the fissile component, and on 29 February 1940, Nier used an instrument he built at the University of Minnesota to separate the world's first uranium-235 sample in the Tate Laboratory. After mailed to Columbia University's cyclotron, John Dunning confirmed the sample to be the isolated fissile material on 1 March. Further work found that the far more common uranium-238 isotope can be transmuted into plutonium, which, like uranium-235, is also fissile by thermal neutrons. These discoveries led numerous countries to begin working on the development of nuclear weapons and nuclear power. Despite fission having been discovered in Germany, the ''Uranverein
The Uranverein ( en, "Uranium Club") or Uranprojekt ( en, "Uranium Project") was the name given to the project in Germany to research nuclear technology, including nuclear weapons and nuclear reactors, during World War II. It went through s ...
'' ("uranium club") Germany's wartime project to research nuclear power and/or weapons was hampered by limited resources, infighting, the exile or non-involvement of several prominent scientists in the field and several crucial mistakes such as failing to account for impurities in available graphite samples which made it appear less suitable as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
than it is in reality. Germany's attempts to build a natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes fr ...
/ heavy water reactor had not come close to reaching criticality by the time the Americans reached Haigerloch, the site of the last German wartime reactor experiment.
On 2 December 1942, as part of the Manhattan Project, another team led by Enrico Fermi was able to initiate the first artificial self-sustained nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
, Chicago Pile-1
Chicago Pile-1 (CP-1) was the world's first artificial nuclear reactor. On 2 December 1942, the first human-made self-sustaining nuclear chain reaction was initiated in CP-1, during an experiment led by Enrico Fermi. The secret development of t ...
. An initial plan using enriched uranium-235 was abandoned as it was as yet unavailable in sufficient quantities. Working in a lab below the stands of Stagg Field
Amos Alonzo Stagg Field is the name of two successive football fields for the University of Chicago. Beyond sports, the first Stagg Field (1893–1957) is remembered for its role in a landmark scientific achievement of Enrico Fermi and the Metall ...
at the University of Chicago, the team created the conditions needed for such a reaction by piling together 400 short tons (360 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United States ...
) of graphite, 58 short tons (53 metric tons) of uranium oxide, and six short tons (5.5 metric tons) of uranium metal, a majority of which was supplied by Westinghouse Lamp Plant in a makeshift production process.
Nuclear weaponry
 Two major types of atomic bombs were developed by the United States during World War II: a uranium-based device (codenamed " Little Boy") whose fissile material was highly enriched uranium, and a plutonium-based device (see Trinity test and " Fat Man") whose plutonium was derived from uranium-238. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the
Two major types of atomic bombs were developed by the United States during World War II: a uranium-based device (codenamed " Little Boy") whose fissile material was highly enriched uranium, and a plutonium-based device (see Trinity test and " Fat Man") whose plutonium was derived from uranium-238. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
ese city of Hiroshima
is the capital of Hiroshima Prefecture in Japan. , the city had an estimated population of 1,199,391. The gross domestic product (GDP) in Greater Hiroshima, Hiroshima Urban Employment Area, was US$61.3 billion as of 2010. Kazumi Matsui h ...
on 6 August 1945. Exploding with a yield equivalent to 12,500 tonnes of TNT, the blast and thermal wave of the bomb destroyed nearly 50,000 buildings and killed approximately 75,000 people (see Atomic bombings of Hiroshima and Nagasaki
The United States detonated two atomic bombs over the Japanese cities of Hiroshima and Nagasaki on 6 and 9 August 1945, respectively. The two bombings killed between 129,000 and 226,000 people, most of whom were civilians, and remain the onl ...
). Initially it was believed that uranium was relatively rare, and that nuclear proliferation
Nuclear proliferation is the spread of nuclear weapons, fissionable material, and weapons-applicable nuclear technology and information to nations not recognized as " Nuclear Weapon States" by the Treaty on the Non-Proliferation of Nuclear Wea ...
could be avoided by simply buying up all known uranium stocks, but within a decade large deposits of it were discovered in many places around the world.
Reactors
 The X-10 Graphite Reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, formerly known as the Clinton Pile and X-10 Pile, was the world's second artificial nuclear reactor (after Enrico Fermi's Chicago Pile) and was the first reactor designed and built for continuous operation.
The X-10 Graphite Reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, formerly known as the Clinton Pile and X-10 Pile, was the world's second artificial nuclear reactor (after Enrico Fermi's Chicago Pile) and was the first reactor designed and built for continuous operation. Argonne National Laboratory
Argonne National Laboratory is a science and engineering research United States Department of Energy National Labs, national laboratory operated by University of Chicago, UChicago Argonne LLC for the United States Department of Energy. The facil ...
's Experimental Breeder Reactor I, located at the Atomic Energy Commission's National Reactor Testing Station near Arco, Idaho, became the first nuclear reactor to create electricity on 20 December 1951. Initially, four 150-watt light bulbs were lit by the reactor, but improvements eventually enabled it to power the whole facility (later, the town of Arco became the first in the world to have all its electricity come from nuclear power generated by BORAX-III
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.
, another reactor designed and operated by Argonne National Laboratory
Argonne National Laboratory is a science and engineering research United States Department of Energy National Labs, national laboratory operated by University of Chicago, UChicago Argonne LLC for the United States Department of Energy. The facil ...
). The world's first commercial scale nuclear power station, Obninsk in the Soviet Union, began generation with its reactor AM-1 on 27 June 1954. Other early nuclear power plants were Calder Hall in England, which began generation on 17 October 1956, and the Shippingport Atomic Power Station in Pennsylvania, which began on 26 May 1958. Nuclear power was used for the first time for propulsion by a submarine
A submarine (or sub) is a watercraft capable of independent operation underwater. It differs from a submersible, which has more limited underwater capability. The term is also sometimes used historically or colloquially to refer to remotely op ...
, the USS ''Nautilus'', in 1954.
Prehistoric naturally occurring fission
In 1972, the French physicistFrancis Perrin Francis Perrin may refer to:
* Francis Perrin (actor) (born 1947), French actor, screenwriter and director
* Francis Perrin (physicist) (1901–1992), French physicist
See also
* Perrin (disambiguation)
{{hndis, Perrin, Francis ...
discovered fifteen ancient and no longer active natural nuclear fission reactors in three separate ore deposits at the Oklo mine in Gabon, West Africa, collectively known as the Oklo Fossil Reactors
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. Th ...
. The ore deposit is 1.7 billion years old; then, uranium-235 constituted about 3% of the total uranium on Earth. This is high enough to permit a sustained nuclear fission chain reaction to occur, provided other supporting conditions exist. The capacity of the surrounding sediment to contain the nuclear waste products has been cited by the U.S. federal government as supporting evidence for the feasibility to store spent nuclear fuel at the Yucca Mountain nuclear waste repository.
Contamination and the Cold War legacy
Navajo
The Navajo (; British English: Navaho; nv, Diné or ') are a Native American people of the Southwestern United States.
With more than 399,494 enrolled tribal members , the Navajo Nation is the largest federally recognized tribe in the United ...
uranium miners, for example, has been documented and linked to their occupation. The Radiation Exposure Compensation Act, a 1990 law in the US, required $100,000 in "compassion payments" to uranium miners diagnosed with cancer or other respiratory ailments.
During the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because the ...
between the Soviet Union and the United States, huge stockpiles of uranium were amassed and tens of thousands of nuclear weapons were created using enriched uranium and plutonium made from uranium. Since the break-up of the Soviet Union
The dissolution of the Soviet Union, also negatively connoted as rus, Разва́л Сове́тского Сою́за, r=Razvál Sovétskogo Soyúza, ''Ruining of the Soviet Union''. was the process of internal disintegration within the Sov ...
in 1991, an estimated 600 short tons (540 metric tons) of highly enriched weapons grade uranium (enough to make 40,000 nuclear warheads) have been stored in often inadequately guarded facilities in the Russian Federation and several other former Soviet states. Police in Asia, Europe, and South America on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources. From 1993 to 2005 the Material Protection, Control, and Accounting Program, operated by the federal government of the United States, spent approximately US $550 million to help safeguard uranium and plutonium stockpiles in Russia. This money was used for improvements and security enhancements at research and storage facilities. ''Scientific American'' reported in February 2006 that in some of the facilities security consisted of chain link fences which were in severe states of disrepair. According to an interview from the article, one facility had been storing samples of enriched (weapons grade) uranium in a broom closet before the improvement project; another had been keeping track of its stock of nuclear warheads using index cards kept in a shoe box.
Occurrence
Origin
Along with all elements having atomic weights higher than that of iron, uranium is only naturally formed by ther-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
(rapid neutron capture) in supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
e and neutron star mergers. Primordial thorium and uranium are only produced in the r-process, because the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
(slow neutron capture) is too slow and cannot pass the gap of instability after bismuth. Besides the two extant primordial uranium isotopes, 235U and 238U, the r-process also produced significant quantities of 236U, which has a shorter half-life and so is an extinct radionuclide, having long since decayed completely to 232Th. Uranium-236 was itself enriched by the decay of 244Pu, accounting for the observed higher-than-expected abundance of thorium and lower-than-expected abundance of uranium. While the natural abundance of uranium has been supplemented by the decay of extinct 242Pu (half-life 0.375 million years) and 247Cm (half-life 16 million years), producing 238U and 235U respectively, this occurred to an almost negligible extent due to the shorter half-lives of these parents and their lower production than 236U and 244Pu, the parents of thorium: the 247Cm:235U ratio at the formation of the Solar System was .
Biotic and abiotic

mantle
A mantle is a piece of clothing, a type of cloak. Several other meanings are derived from that.
Mantle may refer to:
*Mantle (clothing), a cloak-like garment worn mainly by women as fashionable outerwear
**Mantle (vesture), an Eastern Orthodox ve ...
is thought to be the main source of heat that keeps the Earth's outer core in the liquid state and drives mantle convection, which in turn drives plate tectonics.
Uranium's average concentration in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
is (depending on the reference) 2 to 4 parts per million, or about 40 times as abundant as silver. The Earth's crust from the surface to 25 km (15 mi) down is calculated to contain 1017 kg (2 lb) of uranium while the oceans may contain 1013 kg (2 lb). The concentration of uranium in soil ranges from 0.7 to 11 parts per million (up to 15 parts per million in farmland soil due to use of phosphate fertilizers), and its concentration in sea water is 3 parts per billion.
Uranium is more plentiful than antimony, tin, cadmium, mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, or silver, and it is about as abundant as arsenic or molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
. Uranium is found in hundreds of minerals, including uraninite (the most common uranium ore), carnotite, autunite, uranophane, torbernite, and coffinite. Significant concentrations of uranium occur in some substances such as phosphate rock deposits, and minerals such as lignite
Lignite, often referred to as brown coal, is a soft, brown, combustible, sedimentary rock formed from naturally compressed peat. It has a carbon content around 25–35%, and is considered the lowest rank of coal due to its relatively low heat ...
, and monazite sands in uranium-rich ores (it is recovered commercially from sources with as little as 0.1% uranium).
 Some bacteria, such as '' Shewanella putrefaciens'', '' Geobacter metallireducens'' and some strains of ''
Some bacteria, such as '' Shewanella putrefaciens'', '' Geobacter metallireducens'' and some strains of ''Burkholderia fungorum
Paraburkholderia fungorum (P. fungorum) is a Gram-negative species of bacteria. that has been commonly used as a beneficial microorganism in agriculture as an agent for biocontrol and bioremediation. ''Paraburkholderia fungorum'' is Some of its ...
'', use uranium for their growth and convert U(VI) to U(IV). Recent research suggests that this pathway includes reduction of the soluble U(VI) via an intermediate U(V) pentavalent state.
Other organisms, such as the lichen ''Trapelia involuta'' or microorganisms such as the bacterium ''Citrobacter
''Citrobacter'' is a genus of Gram-negative coliform bacteria in the family Enterobacteriaceae.
The species ''C. amalonaticus'', ''C. koseri'', and ''C. freundii'' can use citrate as a sole carbon source. ''Citrobacter'' species are differentia ...
'', can absorb concentrations of uranium that are up to 300 times the level of their environment. ''Citrobacter'' species absorb uranyl ions when given glycerol phosphate (or other similar organic phosphates). After one day, one gram of bacteria can encrust themselves with nine grams of uranyl phosphate crystals; this creates the possibility that these organisms could be used in bioremediation to decontaminate
Decontamination (sometimes abbreviated as decon, dcon, or decontam) is the process of removing contaminants on an object or area, including chemicals, micro-organisms or radioactive substances. This may be achieved by chemical reaction, disinfecti ...
uranium-polluted water.
The proteobacterium '' Geobacter'' has also been shown to bioremediate uranium in ground water. The mycorrhizal fungus Glomus intraradices increases uranium content in the roots of its symbiotic plant.
In nature, uranium(VI) forms highly soluble carbonate complexes at alkaline pH. This leads to an increase in mobility and availability of uranium to groundwater and soil from nuclear wastes which leads to health hazards. However, it is difficult to precipitate uranium as phosphate in the presence of excess carbonate at alkaline pH. A '' Sphingomonas'' sp. strain BSAR-1 has been found to express a high activity alkaline phosphatase (PhoK) that has been applied for bioprecipitation of uranium as uranyl phosphate species from alkaline solutions. The precipitation ability was enhanced by overexpressing PhoK protein in ''E. coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
''.
Plants absorb some uranium from soil. Dry weight concentrations of uranium in plants range from 5 to 60 parts per billion, and ash from burnt wood can have concentrations up to 4 parts per million. Dry weight concentrations of uranium in food
Food is any substance consumed by an organism for nutritional support. Food is usually of plant, animal, or fungal origin, and contains essential nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals. The substance is inge ...
plants are typically lower with one to two micrograms per day ingested through the food people eat.
Production and mining
Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
(6,350 t), Niger (4,518 t), Namibia (4,323 t) and Russia (3,135 t).
Uranium ore is mined in several ways: by open pit, underground, in-situ leaching, and borehole mining (see uranium mining). Low-grade uranium ore mined typically contains 0.01 to 0.25% uranium oxides. Extensive measures must be employed to extract the metal from its ore.. High-grade ores found in Athabasca Basin deposits in Saskatchewan, Canada can contain up to 23% uranium oxides on average. Uranium ore is crushed and rendered into a fine powder and then leached with either an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
or alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
. The leachate
A leachate is any liquid that, in the course of passing through matter, extracts soluble or suspended solids, or any other component of the material through which it has passed.
Leachate is a widely used term in the environmental sciences wher ...
is subjected to one of several sequences of precipitation, solvent extraction, and ion exchange. The resulting mixture, called yellowcake, contains at least 75% uranium oxides U3O8. Yellowcake is then calcined to remove impurities from the milling process before refining and conversion.
Commercial-grade uranium can be produced through the reduction of uranium halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s with alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
or alkaline earth metals. Uranium metal can also be prepared through electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of or
, dissolved in molten calcium chloride () and sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
( NaCl) solution. Very pure uranium is produced through the thermal decomposition of uranium halides on a hot filament.
Resources and reserves
 It is estimated that 5.5 million tonnes of uranium exists in ore reserves that are economically viable at US$59 per lb of uranium, while 35 million tonnes are classed as mineral resources (reasonable prospects for eventual economic extraction). Prices went from about $10/lb in May 2003 to $138/lb in July 2007. This has caused a big increase in spending on exploration, with US$200 million being spent worldwide in 2005, a 54% increase on the previous year. This trend continued through 2006, when expenditure on exploration rocketed to over $774 million, an increase of over 250% compared to 2004. The OECD Nuclear Energy Agency said exploration figures for 2007 would likely match those for 2006.
Australia has 31% of the world's known uranium ore reserves and the world's largest single uranium deposit, located at the Olympic Dam Mine in South Australia. There is a significant reserve of uranium in Bakouma, a
It is estimated that 5.5 million tonnes of uranium exists in ore reserves that are economically viable at US$59 per lb of uranium, while 35 million tonnes are classed as mineral resources (reasonable prospects for eventual economic extraction). Prices went from about $10/lb in May 2003 to $138/lb in July 2007. This has caused a big increase in spending on exploration, with US$200 million being spent worldwide in 2005, a 54% increase on the previous year. This trend continued through 2006, when expenditure on exploration rocketed to over $774 million, an increase of over 250% compared to 2004. The OECD Nuclear Energy Agency said exploration figures for 2007 would likely match those for 2006.
Australia has 31% of the world's known uranium ore reserves and the world's largest single uranium deposit, located at the Olympic Dam Mine in South Australia. There is a significant reserve of uranium in Bakouma, a sub-prefecture
A subprefecture is an administrative division of a country that is below prefecture or province.
Albania
There are twelve Albanian counties or prefectures, each of which is divided into several districts, sometimes translated as subprefecture ...
in the prefecture
A prefecture (from the Latin ''Praefectura'') is an administrative jurisdiction traditionally governed by an appointed prefect. This can be a regional or local government subdivision in various countries, or a subdivision in certain international ...
of Mbomou in the Central African Republic.
Some nuclear fuel comes from nuclear weapons being dismantled, such as from the Megatons to Megawatts Program.
An additional 4.6 billion tonnes of uranium are estimated to be dissolved in sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
(Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
ese scientists in the 1980s showed that extraction of uranium from sea water using ion exchangers was technically feasible). There have been experiments to extract uranium from sea water, but the yield has been low due to the carbonate present in the water. In 2012, ORNL
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
researchers announced the successful development of a new absorbent material dubbed HiCap which performs surface retention of solid or gas molecules, atoms or ions and also effectively removes toxic metals from water, according to results verified by researchers at Pacific Northwest National Laboratory.
Supplies
 In 2005, seventeen countries produced concentrated uranium oxides: Canada (27.9% of world production),
In 2005, seventeen countries produced concentrated uranium oxides: Canada (27.9% of world production), Australia
Australia, officially the Commonwealth of Australia, is a Sovereign state, sovereign country comprising the mainland of the Australia (continent), Australian continent, the island of Tasmania, and numerous List of islands of Australia, sma ...
(22.8%), Kazakhstan (10.5%), Russia (8.0%), Namibia (7.5%), Niger (7.4%), Uzbekistan (5.5%), the United States (2.5%), Argentina (2.1%), Ukraine (1.9%) and China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
(1.7%). In 2008 Kazakhstan was forecast to increase production and may have become the world's largest producer of uranium by 2009 with an expected production of 12,826 tonnes, compared to Canada with 11,100 t and Australia with 9,430 t. The predictions have come true. In 2019 Kazakhstan produces the largest share of uranium from mines 42% of world supply, followed by Canada (13%) and Australia (12%), Namibia (10%), Uzbekistan (6%), Niger (5%), Russia (5%), China (3%), Ukraine (1.5%), USA (0.12%), India (0.6%), Iran (0.13%), with total world production 54752 tonnes from mines. However, it should be mentioned that in 2019 uranium was mined not only by conventional underground mining of ores 43% of production (54752 tonnes), where rock mineralised is removed from the ground, breaking it up and treating it to remove the minerals being sought but also by in-situ leaching methods (ISL) 57% of world production (64,566 tonnes).
In the late 1960s, UN geologists also discovered major uranium deposits and other rare mineral reserves in Somalia. The find was the largest of its kind, with industry experts estimating the deposits at over 25% of the world's then known uranium reserves of 800,000 tons.
The ultimate available supply is believed to be sufficient for at least the next 85 years, although some studies indicate underinvestment in the late twentieth century may produce supply problems in the 21st century.
Uranium deposits seem to be log-normal distributed. There is a 300-fold increase in the amount of uranium recoverable for each tenfold decrease in ore grade.
In other words, there is little high grade ore and proportionately much more low grade ore available.
Compounds
Oxidation states and oxides
Oxides
Calcined uranium yellowcake, as produced in many large mills, contains a distribution of uranium oxidation species in various forms ranging from most oxidized to least oxidized. Particles with short residence times in a calciner will generally be less oxidized than those with long retention times or particles recovered in the stack scrubber. Uranium content is usually referenced to , which dates to the days of the Manhattan Project when was used as an analytical chemistry reporting standard. Phase relationships in the uranium-oxygen system are complex. The most important oxidation states of uranium are uranium(IV) and uranium(VI), and their two correspondingoxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s are, respectively, uranium dioxide () and uranium trioxide ().. Other uranium oxides such as uranium monoxide (UO), diuranium pentoxide (), and uranium peroxide () also exist.
The most common forms of uranium oxide are triuranium octoxide () and . Both oxide forms are solids that have low solubility in water and are relatively stable over a wide range of environmental conditions. Triuranium octoxide is (depending on conditions) the most stable compound of uranium and is the form most commonly found in nature. Uranium dioxide is the form in which uranium is most commonly used as a nuclear reactor fuel. At ambient temperatures, will gradually convert to . Because of their stability, uranium oxides are generally considered the preferred chemical form for storage or disposal.
Aqueous chemistry
 Salts of many oxidation states of uranium are water- soluble and may be studied in
Salts of many oxidation states of uranium are water- soluble and may be studied in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
s. The most common ionic forms are (brown-red), (green), (unstable), and (yellow), for U(III), U(IV), U(V), and U(VI), respectively.. A few solid and semi-metallic compounds such as UO and US exist for the formal oxidation state uranium(II), but no simple ions are known to exist in solution for that state. Ions of liberate hydrogen from water and are therefore considered to be highly unstable. The ion represents the uranium(VI) state and is known to form compounds such as uranyl carbonate, uranyl chloride and uranyl sulfate. also forms complexes with various organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
chelating agents, the most commonly encountered of which is uranyl acetate.
Unlike the uranyl salts of uranium and polyatomic ion uranium-oxide cationic forms, the uranates, salts containing a polyatomic uranium-oxide anion, are generally not water-soluble.
Carbonates
The interactions of carbonate anions with uranium(VI) cause the Pourbaix diagram to change greatly when the medium is changed from water to a carbonate containing solution. While the vast majority of carbonates are insoluble in water (students are often taught that all carbonates other than those of alkali metals are insoluble in water), uranium carbonates are often soluble in water. This is because a U(VI) cation is able to bind two terminal oxides and three or more carbonates to form anionic complexes.Effects of pH
The uranium fraction diagrams in the presence of carbonate illustrate this further: when the pH of a uranium(VI) solution increases, the uranium is converted to a hydrated uranium oxide hydroxide and at high pHs it becomes an anionic hydroxide complex. When carbonate is added, uranium is converted to a series of carbonate complexes if the pH is increased. One effect of these reactions is increased solubility of uranium in the pH range 6 to 8, a fact that has a direct bearing on the long term stability of spent uranium dioxide nuclear fuels.Hydrides, carbides and nitrides
Uranium metal heated to reacts with hydrogen to form uranium hydride. Even higher temperatures will reversibly remove the hydrogen. This property makes uranium hydrides convenient starting materials to create reactive uranium powder along with various uranium carbide, nitride, andhalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
compounds.. Two crystal modifications of uranium hydride exist: an α form that is obtained at low temperatures and a β form that is created when the formation temperature is above 250 °C.
Uranium carbides and uranium nitrides are both relatively inert
Inert may refer to:
* Chemically inert, not chemically reactive
** Inert gas
** Noble gas, historically called inert gas
* Inert knowledge, information which one can express but not use
* Inert waste, waste which is neither chemically nor biol ...
semimetallic compounds that are minimally soluble in acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s, react with water, and can ignite in air to form . Carbides of uranium include uranium monocarbide (U C), uranium dicarbide (), and diuranium tricarbide (). Both UC and are formed by adding carbon to molten uranium or by exposing the metal to carbon monoxide at high temperatures. Stable below 1800 °C, is prepared by subjecting a heated mixture of UC and to mechanical stress.. Uranium nitrides obtained by direct exposure of the metal to nitrogen include uranium mononitride (UN), uranium dinitride (), and diuranium trinitride ().
Halides
 All uranium fluorides are created using uranium tetrafluoride (); itself is prepared by hydrofluorination of uranium dioxide. Reduction of with hydrogen at 1000 °C produces uranium trifluoride (). Under the right conditions of temperature and pressure, the reaction of solid with gaseous uranium hexafluoride () can form the intermediate fluorides of , , and .
At room temperatures, has a high vapor pressure, making it useful in the gaseous diffusion process to separate the rare uranium-235 from the common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:
: + 4 HF → + 2 (500 °C, endothermic)
: + → (350 °C, endothermic)
The resulting , a white solid, is highly reactive (by fluorination), easily
All uranium fluorides are created using uranium tetrafluoride (); itself is prepared by hydrofluorination of uranium dioxide. Reduction of with hydrogen at 1000 °C produces uranium trifluoride (). Under the right conditions of temperature and pressure, the reaction of solid with gaseous uranium hexafluoride () can form the intermediate fluorides of , , and .
At room temperatures, has a high vapor pressure, making it useful in the gaseous diffusion process to separate the rare uranium-235 from the common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:
: + 4 HF → + 2 (500 °C, endothermic)
: + → (350 °C, endothermic)
The resulting , a white solid, is highly reactive (by fluorination), easily sublimes
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point i ...
(emitting a vapor that behaves as a nearly ideal gas), and is the most volatile compound of uranium known to exist.
One method of preparing uranium tetrachloride () is to directly combine chlorine with either uranium metal or uranium hydride. The reduction of by hydrogen produces uranium trichloride () while the higher chlorides of uranium are prepared by reaction with additional chlorine. All uranium chlorides react with water and air.
Bromides and iodides of uranium are formed by direct reaction of, respectively, bromine and iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
with uranium or by adding to those element's acids. Known examples include: , , , and . has never been prepared. Uranium oxyhalides are water-soluble and include , , , and . Stability of the oxyhalides decrease as the atomic weight of the component halide increases.
Isotopes
Natural concentrations
Natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes fr ...
consists of three major isotopes: uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
(99.28% natural abundance), uranium-235 (0.71%), and uranium-234 (0.0054%). All three are radioactive, emitting alpha particles, with the exception that all three of these isotopes have small probabilities of undergoing spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdo ...
. There are also four other trace isotopes: uranium-239, which is formed when 238U undergoes spontaneous fission, releasing neutrons that are captured by another 238U atom; uranium-237, which is formed when 238U captures a neutron but emits two more, which then decays to neptunium-237; uranium-236, which occurs in trace quantities due to neutron capture on 235U and as a decay product of plutonium-244; and finally, uranium-233, which is formed in the decay chain of neptunium-237. It is also expected that thorium-232 should be able to undergo double beta decay, which would produce uranium-232, but this has not yet been observed experimentally. The only significant deviation from the U-235 to U-238 ratio in any known natural samples occurs in Oklo, Gabon where natural nuclear fission reactors consumed some of the U-235 some two billion years ago when the ratio of U-235 to U-238 was more akin to that of low enriched uranium allowing regular ("light") water to act as a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely mo ...
akin to the process in humanmade light water reactors. The existence of such natural fission reactors which had been theoretically predicted beforehand was proven as the slight deviation of U-235 concentration from the expected values were discovered during uranium enrichment in France. Subsequent investigations to rule out any nefarious human action (such as stealing of U-235) confirmed the theory by finding isotope ratios of common fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s (or rather their stable daughter nuclides) in line with the values expected for fission but deviating from the values expected for non-fission derived samples of those elements.
Uranium-238 is the most stable isotope of uranium, with a half-life of about 4.468 years, roughly the age of the Earth
The age of Earth is estimated to be 4.54 ± 0.05 billion years This age may represent the age of Earth's accretion, or core formation, or of the material from which Earth formed. This dating is based on evidence from radiometric age-dating of ...
. Uranium-235 has a half-life of about 7.13 years, and uranium-234 has a half-life of about 2.48 years.. Uranium-238 is usually an alpha emitter (occasionally, it undergoes spontaneous fission), decaying through the uranium series, which has 18 members, into lead-206, by a variety of different decay paths.
The decay chain of 235U, which is called the actinium series, has 15 members and eventually decays into lead-207. The constant rates of decay in these decay series makes the comparison of the ratios of parent to daughter elements useful in radiometric dating.
Uranium-234, which is a member of the uranium series (the decay chain of uranium-238), decays to lead-206 through a series of relatively short-lived isotopes.
Uranium-233 is made from thorium-232 by neutron bombardment, usually in a nuclear reactor, and 233U is also fissile. Its decay chain forms part of the neptunium series and ends at bismuth-209 and thallium-205.
Uranium-235 is important for both nuclear reactors and nuclear weapons, because it is the only uranium isotope existing in nature on Earth in any significant amount that is fissile. This means that it can be split into two or three fragments (fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
) by thermal neutrons.
Uranium-238 is not fissile, but is a fertile isotope, because after neutron activation it can be converted to plutonium-239, another fissile isotope. Indeed, the 238U nucleus can absorb one neutron to produce the radioactive isotope uranium-239. 239U decays by beta emission to neptunium-239, also a beta-emitter, that decays in its turn, within a few days into plutonium-239. 239Pu was used as fissile material in the first atomic bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions (thermonuclear bomb), producing a nuclear explosion. Both bomb ...
detonated in the "Trinity test" on 15 July 1945 in New Mexico.
Enrichment
 In nature, uranium is found as uranium-238 (99.2742%) and uranium-235 (0.7204%). Isotope separation concentrates (enriches) the fissionable uranium-235 for nuclear weapons and most nuclear power plants, except for gas cooled reactors and pressurised heavy water reactors. Most neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the
In nature, uranium is found as uranium-238 (99.2742%) and uranium-235 (0.7204%). Isotope separation concentrates (enriches) the fissionable uranium-235 for nuclear weapons and most nuclear power plants, except for gas cooled reactors and pressurised heavy water reactors. Most neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
. The concentration and amount of uranium-235 needed to achieve this is called a ' critical mass'.
To be considered 'enriched', the uranium-235 fraction should be between 3% and 5%. This process produces huge quantities of uranium that is depleted of uranium-235 and with a correspondingly increased fraction of uranium-238, called depleted uranium or 'DU'. To be considered 'depleted', the uranium-235 isotope concentration should be no more than 0.3%. The price of uranium has risen since 2001, so enrichment tailings containing more than 0.35% uranium-235 are being considered for re-enrichment, driving the price of depleted uranium hexafluoride
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium ...
above $130 per kilogram in July 2007 from $5 in 2001.
The gas centrifuge process, where gaseous uranium hexafluoride () is separated by the difference in molecular weight between 235UF6 and 238UF6 using high-speed centrifuges, is the cheapest and leading enrichment process.. The gaseous diffusion process had been the leading method for enrichment and was used in the Manhattan Project. In this process, uranium hexafluoride is repeatedly diffused
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
through a silver- zinc membrane, and the different isotopes of uranium are separated by diffusion rate (since uranium-238 is heavier it diffuses slightly slower than uranium-235). The molecular laser isotope separation method employs a laser beam of precise energy to sever the bond between uranium-235 and fluorine. This leaves uranium-238 bonded to fluorine and allows uranium-235 metal to precipitate from the solution. An alternative laser method of enrichment is known as atomic vapor laser isotope separation
Atomic vapor laser isotope separation, or AVLIS, is a method by which specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions. A similar technology, using molecules instead of atoms, is ...
(AVLIS) and employs visible tunable lasers such as dye lasers. Another method used is liquid thermal diffusion.
Human exposure
A person can be exposed to uranium (or its radioactive daughters, such as radon) by inhaling dust in air or by ingesting contaminated water and food. The amount of uranium in air is usually very small; however, people who work in factories that process phosphate fertilizers, live near government facilities that made or tested nuclear weapons, live or work near a modern battlefield where depleted uranium weapons have been used, or live or work near a coal-fired power plant, facilities that mine or process uranium ore, or enrich uranium for reactor fuel, may have increased exposure to uranium. Houses or structures that are over uranium deposits (either natural or man-made slag deposits) may have an increased incidence of exposure to radon gas. The Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit for uranium exposure in the workplace as 0.25 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.2 mg/m3 over an 8-hour workday and a short-term limit of 0.6 mg/m3. At levels of 10 mg/m3, uranium is immediately dangerous to life and health. Most ingested uranium is excreted during digestion. Only 0.5% is absorbed when insoluble forms of uranium, such as its oxide, are ingested, whereas absorption of the more soluble uranyl ion can be up to 5%. However, soluble uranium compounds tend to quickly pass through the body, whereas insoluble uranium compounds, especially when inhaled by way of dust into thelung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of t ...
s, pose a more serious exposure hazard. After entering the bloodstream, the absorbed uranium tends to bioaccumulate and stay for many years in bone tissue because of uranium's affinity for phosphates. Uranium is not absorbed through the skin, and alpha particles released by uranium cannot penetrate the skin.
Incorporated uranium becomes uranyl ions, which accumulate in bone, liver, kidney, and reproductive tissues. Uranium can be decontaminated from steel surfaces and aquifers.
Effects and precautions
Normal functioning of the kidney, brain, liver, heart, and other systems can be affected by uranium exposure, because, besides being weakly radioactive, uranium is a toxic metal. Uranium is also areproductive toxicant
Reproductive toxicity refers to the potential risk from a given chemical, physical or biologic agent to adversely affect both male and female fertility as well as offspring development. Reproductive toxicants may adversely affect sexual function ...
. Radiological effects are generally local because alpha radiation, the primary form of 238U decay, has a very short range, and will not penetrate skin. Alpha radiation from inhaled uranium has been demonstrated to cause lung cancer in exposed nuclear workers. Uranyl () ions, such as from uranium trioxide or uranyl nitrate and other hexavalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
uranium compounds, have been shown to cause birth defects and immune system damage in laboratory animals. While the CDC has published one study that no human cancer has been seen as a result of exposure to natural or depleted uranium, exposure to uranium and its decay products, especially radon, are widely known and significant health threats. Exposure to strontium-90, iodine-131, and other fission products is unrelated to uranium exposure, but may result from medical procedures or exposure to spent reactor fuel or fallout from nuclear weapons.
Although accidental inhalation exposure to a high concentration of uranium hexafluoride has
resulted in human fatalities, those deaths were associated with the generation of highly toxic hydrofluoric acid and uranyl fluoride rather than with uranium itself. Finely divided uranium metal presents a fire hazard because uranium is pyrophoric; small grains will ignite spontaneously in air at room temperature.
Uranium metal is commonly handled with gloves as a sufficient precaution. Uranium concentrate is handled and contained so as to ensure that people do not inhale or ingest it.
See also
* K-65 residues * List of countries by uranium production *List of countries by uranium reserves
Uranium reserves are reserves of recoverable uranium, regardless of isotope, based on a set market price. The list given here is based on ''Uranium 2020: Resources, Production and Demand'', a joint report by the OECD Nuclear Energy Agency and th ...
* List of uranium projects
* Lists of nuclear disasters and radioactive incidents
* Nuclear and radiation accidents and incidents
* Nuclear engineering
Nuclear engineering is the branch of engineering concerned with the application of breaking down atomic nuclei ( fission) or of combining atomic nuclei (fusion), or with the application of other sub-atomic processes based on the principles of n ...
* Nuclear fuel cycle
* Nuclear physics
* Quintuple bond (earlier thought to be a Phi bond
In chemistry, phi bonds (φ bonds) are covalent chemical bonds, where six lobes of one involved atomic orbital overlap six lobes of the other involved atomic orbital. This overlap leads to the formation of a bonding molecular orbital with three n ...
), in the molecule U2
* Thorium fuel cycle
* World Uranium Hearing
The World Uranium Hearing was held in Salzburg, Austria in September 1992. Anti-nuclear speakers from all continents, including indigenous speakers and scientists, testified to the health and environmental problems of uranium mining and processing ...
Notes
References
* *External links
U.S. EPA: Radiation Information for Uranium
from
World Nuclear Association
World Nuclear Association is the international organization that promotes nuclear power and supports the companies that comprise the global nuclear industry. Its members come from all parts of the nuclear fuel cycle, including uranium mining, ur ...
Nuclear fuel data and analysis
from the
U.S. Energy Information Administration
The U.S. Energy Information Administration (EIA) is a principal agency of the U.S. Federal Statistical System responsible for collecting, analyzing, and disseminating energy information to promote sound policymaking, efficient markets, and publ ...
Current market price of uranium
Annotated bibliography for uranium from the Alsos Digital Library
NLM Hazardous Substances Databank—Uranium, Radioactive
Mining Uranium at Namibia's Langer Heinrich Mine
World Nuclear News
ATSDR Case Studies in Environmental Medicine: Uranium Toxicity
U.S. Department of Health and Human Services
Uranium
at '' The Periodic Table of Videos'' (University of Nottingham) {{Authority control Chemical elements Actinides Nuclear fuels Nuclear materials Suspected male-mediated teratogens Manhattan Project