|
Plutonium-244
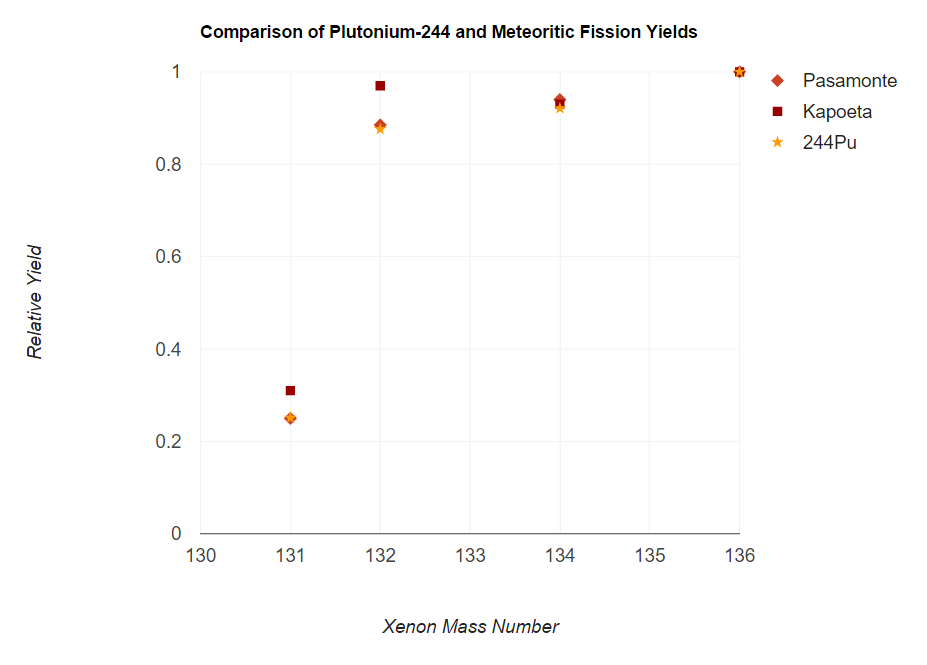
Plutonium-244 (Pu) is an isotope of plutonium that has a half-life of 81.3 million years. This is longer than any other isotope of plutonium and longer than any other known isotope of an element beyond bismuth, except for the three naturally abundant ones: uranium-235 (704 million years), uranium-238 (4.468 billion years), and thorium-232 (14.05 billion years). Given the half-life of Pu, an exceedingly small amount should still be present on Earth, making plutonium a likely but unproven candidate as the shortest-lived primordial element. Natural occurrence Accurate measurements, beginning in the early 1970s, appeared to detect primordial plutonium-244, making it the shortest-lived primordial nuclide. The amount of Pu in the pre-Solar nebula (4.57×10 years ago) was estimated as 0.8% the amount of U. As the age of the Earth is about 56 half-lives of Pu, the amount of Pu left should be very small; Hoffman et al. estimated its content in the rare-earth mineral bastnasite as & ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Of Plutonium
Plutonium (Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized before being found in nature, with the first isotope synthesized being Pu in 1940. Twenty-two plutonium radioisotopes have been characterized. The most stable are Pu with a half-life of 81.3 million years; Pu with a half-life of 373,300 years; Pu with a half-life of 24,110 years; and Pu with a half-life of 6,560 years. This element also has eight meta states; all have half-lives of less than one second. The known isotopes of plutonium range from Pu to Pu. The primary decay modes before the most stable isotope, Pu, are spontaneous fission and alpha decay; the primary mode after is beta emission. The primary decay products before Pu are isotopes of uranium and neptunium (not considering fission products), and the primary decay products after are is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Element
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the Solar System was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osmium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
R-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy metals, heavier than iron, the "heavy elements", with the other half produced by the p-process and s-process, ''s''-process. The ''r''-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The ''r''-process can typically synthesize the heaviest four isotopes of every heavy element; of these, the heavier two are called ''r-only nuclei'' because they are created exclusively via the ''r''-process. Abundance peaks for the ''r''-process occur near mass numbers (elements Se, Br, and Kr), (elements Te, I, and Xe) and (elements Os, Ir, and Pt). The ''r''-process entails a succession of ''rapid'' neutron captures (hence the name) by one or more heavy Seed nucleus, seed nuclei, typically beginning with nuclei in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutat ... reactions induced by natural radioactivity, such as the production of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supernovae
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original object, called the ''progenitor'', either collapses to a neutron star or black hole, or is completely destroyed to form a diffuse nebula. The peak optical luminosity of a supernova can be comparable to that of an entire galaxy before fading over several weeks or months. The last supernova directly observed in the Milky Way was Kepler's Supernova in 1604, appearing not long after Tycho's Supernova in 1572, both of which were visible to the naked eye. The remnants of more recent supernovae have been found, and observations of supernovae in other galaxies suggest they occur in the Milky Way on average about three times every century. A supernova in the Milky Way would almost certainly be observable through modern astronomical telescopes. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Star Merger
A neutron star merger is the stellar collision of neutron stars. When two neutron stars fall into mutual orbit, they gradually inspiral, spiral inward due to the loss of energy emitted as gravitational radiation. When they finally meet, their merger leads to the formation of either a more massive neutron star, or—if the mass of the remnant exceeds the Tolman–Oppenheimer–Volkoff limit—a black hole. The merger can create a magnetic field that is trillions of times stronger than that of Earth in a matter of one or two milliseconds. The immediate event creates a gamma-ray burst#Short gamma-ray bursts, short gamma-ray burst visible over hundreds of millions, or even billions of light years. The merger of neutron stars momentarily creates an environment of such extreme neutron flux that the r-process, ''r''-process can occur. This reaction accounts for the nucleosynthesis of around half of the isotopes in elements heavier than iron. The mergers also produce kilonovae, which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerator Mass Spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the different methods of mass spectrometry is its ability to separate a rare isotope from an abundant neighboring mass ("abundance sensitivity", e.g. 14C from 12C). The method suppresses molecular isobars completely and in many cases can also separate atomic isobars (e.g. 14N from 14C). This makes possible the detection of naturally occurring, long-lived radio-isotopes such as 10Be, 36Cl, 26Al and 14C. (Their typical isotopic abundance ranges from 10−12 to 10−18.) AMS can outperform the competing technique of decay counting for all isotopes where the half-life is long enough. Other advantages of AMS include its short measuring time as well as its ability to detect atoms in extremely small samples. Method Generally, negative ions are created (atoms are ionized) in an ion source. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bayan Obo
Bayan'obo Mining District ( Mongolian: , zh, s=白云鄂博矿区), or Baiyun-Obo or Baiyun'ebo, is a mining district in the west of Inner Mongolia, China. It is under the administration of Baotou City, the downtown of which is more than to the south. The mines north of the town are the largest deposits of rare-earth elements yet found and, as of 2005, responsible for 45% of global rare-earth element production. In the satellite image at right, vegetation appears red, grassland is light brown, rocks are black, and water surfaces are green. Two circular open-pit mines are visible, as well as a number of tailings ponds and tailings piles. Administrative divisions Bayan Obo Mining District is made up of 2 subdistricts. Climate Economic geology China produced about 81,000 tons of rare-earth metals in 2001; the number jumped to about 120,000 by 2006. According to the Chinese Society of Rare Earths, of waste gas—containing dust concentrate, hydrofluoric acid, sulfur dioxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar System" and "solar system" structures in theinaming guidelines document. The name is commonly rendered in lower case ('solar system'), as, for example, in the ''Oxford English Dictionary'' an''Merriam-Webster's 11th Collegiate Dictionary''. is the gravitationally bound Planetary system, system of the Sun and the objects that orbit it. It Formation and evolution of the Solar System, formed about 4.6 billion years ago when a dense region of a molecular cloud collapsed, forming the Sun and a protoplanetary disc. The Sun is a typical star that maintains a hydrostatic equilibrium, balanced equilibrium by the thermonuclear fusion, fusion of hydrogen into helium at its stellar core, core, releasing this energy from its outer photosphere. As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstellar Medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic medium. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. Although the density of atoms in the ISM is usually far below that in the best laboratory vacuums, the mean free path between collisions is short compared to typical interstellar lengths, so on these scales the ISM behaves as a gas (more precisely, as a Plasma (physics), plasma: it is everywhere at least slightly ionized), responding to pressure forces, and not as a collection of non-interacting particles. The interstellar medium is composed of multiple phases distinguished by whether matter is ionic, atomic, or molecular, and the temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |