|
Uranium-234
Uranium-234 (234U or U-234) is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% (55 parts per million) of the raw uranium because its half-life of just 245,500 years is only about 1/18,000 as long as that of 238U. Thus the rate of to in a natural sample is equivalent to the rate of their half lives to one another. The primary path of production of 234U via nuclear decay is as follows: uranium-238 nuclei emit an alpha particle to become thorium-234. Next, with a short half-life, 234Th nuclei emit a beta particle to become protactinium-234 (234Pa), or more likely a nuclear isomer denoted 234mPa. Finally, 234Pa or 234mPa nuclei emit another beta particle to become 234U nuclei. Uranium-234 nuclei decay by alpha emission to thorium-230, except for the tiny fraction (parts per billion) of nuclei that undergo spontaneous fission. Extraction of rather small amounts of 234U from natura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Uranium
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U (with the exceptions of 220U and 241U). The standard atomic weight of natural uranium is . Naturally occurring uranium is composed of three major isotopes, uranium-238 (99.2739–99.2752% natural abundance), uranium-235 (0.7198–0.7202%), and uranium-234 (0.0050–0.0059%). All three isotopes are radioactive (i.e., they are radioisotopes), and the most abundant and stable is uranium-238, with a half-life of (close to the age of the Earth). Uranium-238 is an alpha emitter, decayi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enriched Uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2739–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7202%), and uranium-234 (234U, 0.0050–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation. There are about 2,000 tonnes of highly enriched uranium in the world, produced mostly for nuclear power, nuclear weapons, naval propulsion, and smaller quantities for research reactors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radioactive isotope of plutonium that has a half-life of 87.7 years. Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suitable for usage in radioisotope thermoelectric generators (RTGs) and radioisotope heater units. The density of plutonium-238 at room temperature is about 19.8 g/cc. The material will generate about 0.57 watts/gram of 238Pu. The bare sphere critical mass of metallic plutonium-238 is not precisely known, but its calculated range is between 9.04 and 10.07 kilograms. History Initial production Plutonium-238 was the first isotope of plutonium to be discovered. It was synthesized by Glenn Seaborg and associates in December 1940 by bombarding uranium-238 with deuterons, creating neptunium-238. The subsequent decay via β− decay creates Plutonium-238. + → + 2 The neptunium isotope then undergoes β− decay to plutonium- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Enrichment
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2739–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7202%), and uranium-234 (234U, 0.0050–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation. There are about 2,000 tonnes of highly enriched uranium in the world, produced mostly for nuclear power, nuclear weapons, naval propulsion, and smaller quantities for research reactors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Emission
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward spontaneo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Engineering
Engineering is the use of scientific method, scientific principles to design and build machines, structures, and other items, including bridges, tunnels, roads, vehicles, and buildings. The discipline of engineering encompasses a broad range of more specialized List of engineering branches, fields of engineering, each with a more specific emphasis on particular areas of applied mathematics, applied science, and types of application. See glossary of engineering. The term ''engineering'' is derived from the Latin ''ingenium'', meaning "cleverness" and ''ingeniare'', meaning "to contrive, devise". Definition The American Engineers' Council for Professional Development (ECPD, the predecessor of Accreditation Board for Engineering and Technology, ABET) has defined "engineering" as: The creative application of scientific principles to design or develop structures, machines, apparatus, or manufacturing processes, or works utilizing them singly or in combination; or to construct o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
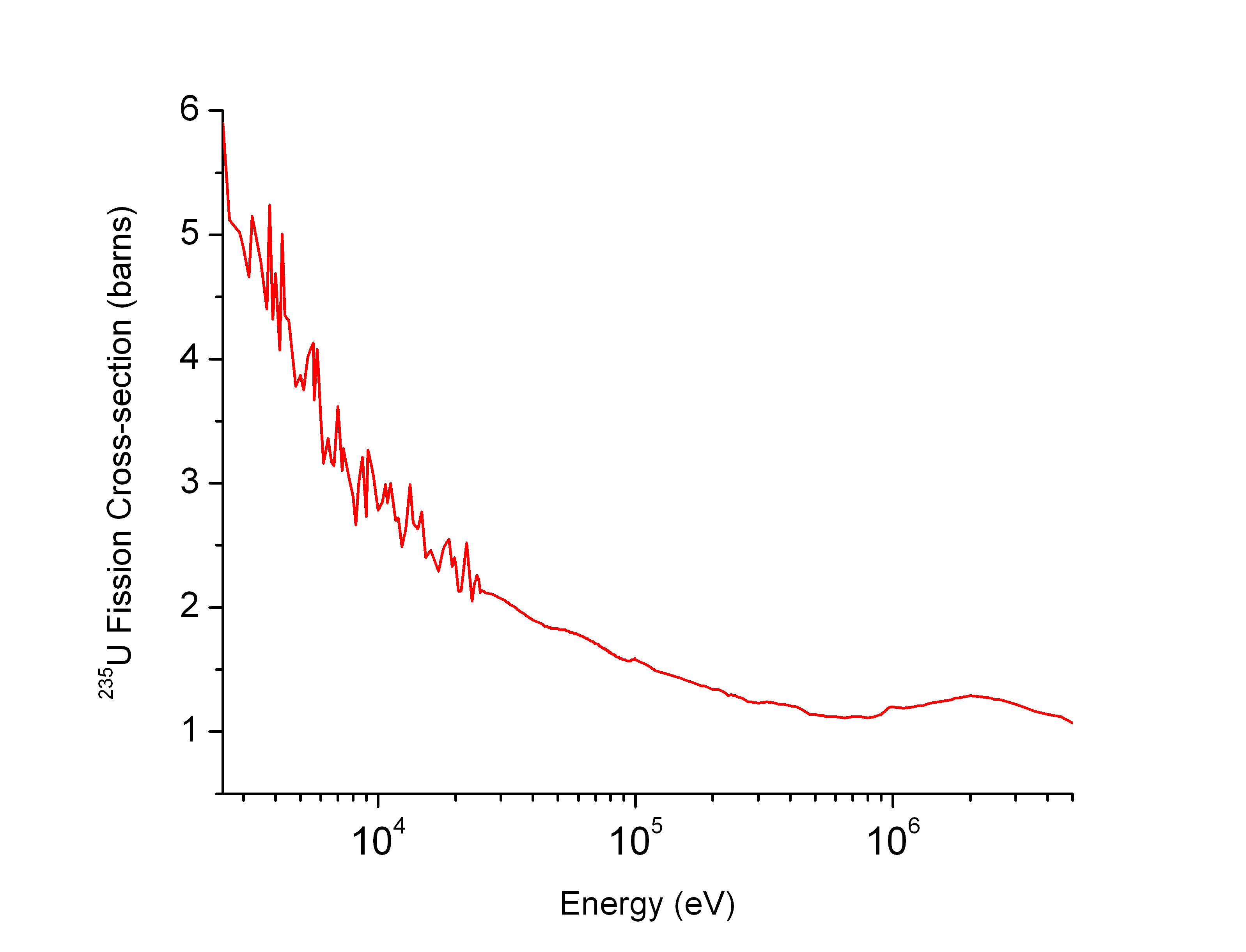
Neutron-capture Cross Section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absorb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years. Nuclear properties The nuclear properties of plutonium-239, as well as the ability to produce large amounts of nearly pure 239Pu more cheaply than highly enriched weapons-grade uranium-235, led to its use in nuclear weapons and nuclear power plants. The fissioning of an atom of uranium-235 in the reactor of a nuclear power plant produces two to three neutrons, and these neutrons can be absorbed by uranium-238 to produce plutonium-239 and other isotopes. Plutonium-239 can also absorb neutrons and fission along with the uranium-235 in a reactor. Of all the common nuclear fuels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Neptunium
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding with neutrons to produce , which then underwent beta decay to . Trace quantities are found in nature from neutron capture reactions by uranium atoms, a fact not discovered until 1951. Twenty-five neptunium radioisotopes have been characterized, with the most stable being with a half-life of 2.14 million years, with a half-life of 154,000 years, and with a half-life of 396.1 days. All of the remaining radioactive isotopes have half-lives that are less than 4.5 days, and the majority of these have half-lives that are less than 50 minutes. This element also has five meta states, with the most stable being (t1/2 22.5 hours). The isotopes of neptunium range from to , t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typified by either slow neutrons (i.e., a thermal system) or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives. Fissile vs fissionable According to the Ronen fissile rule, for a heavy element with 90 ≤ ''Z'' ≤ 100, its isotopes with , with few exceptions, are fissile (where ''N'' = number of neutrons and ''Z'' = number of protons).The fissile rule thus formulated indicates 33 isotopes as likely fissile: Th-225, 227, 229; Pa-228, 230, 232; U-231, 233, 235; Np-234, 236, 238; Pu-237, 239, 241; Am-240, 242, 244; Cm-243, 245, 247; Bk-246, 248, 250; Cf-249, 251, 253; Es-252, 254, 256; Fm-255, 257, 259. Only fourteen (including a long-lived m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reprocessed Uranium
Reprocessed uranium (RepU) is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium makes up the bulk of the material separated during reprocessing. Commercial LWR spent nuclear fuel contains on average (excluding cladding) only four percent plutonium, minor actinides and fission products by weight. Despite it often containing more fissile material than natural uranium, reuse of reprocessed uranium has not been common because of low prices in the uranium market of recent decades, and because it contains undesirable isotopes of uranium. Given sufficiently high uranium prices, it is feasible for reprocessed uranium to be re- enriched and reused. A higher enrichment level is required to compensate for the 236U which is lighter than 238U and therefore concentrates in the enriched product. As enrichment concentrates lighter isotopes on the "enriched" side an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |