Sulfuric Acids on:
[Wikipedia]
[Google]
[Amazon]
Sulfuric acid (
 When allowed to react with superacids, sulfuric acid can act as a base and can be protonated, forming the ion. Salts of have been prepared using the following reaction in liquid HF:
:
The above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply
When allowed to react with superacids, sulfuric acid can act as a base and can be protonated, forming the ion. Salts of have been prepared using the following reaction in liquid HF:
:
The above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply

 Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric
Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric
 Sulfuric acid is a very important commodity chemical, and indeed, a nation's sulfuric acid production is a good indicator of its industrial strength. World production in the year 2004 was about 180 million
Sulfuric acid is a very important commodity chemical, and indeed, a nation's sulfuric acid production is a good indicator of its industrial strength. World production in the year 2004 was about 180 million
 Sulfuric acid acts as the electrolyte in lead–acid batteries (lead-acid accumulator):
At
Sulfuric acid acts as the electrolyte in lead–acid batteries (lead-acid accumulator):
At  Overall:
:
Overall:
:
 The study of
The study of
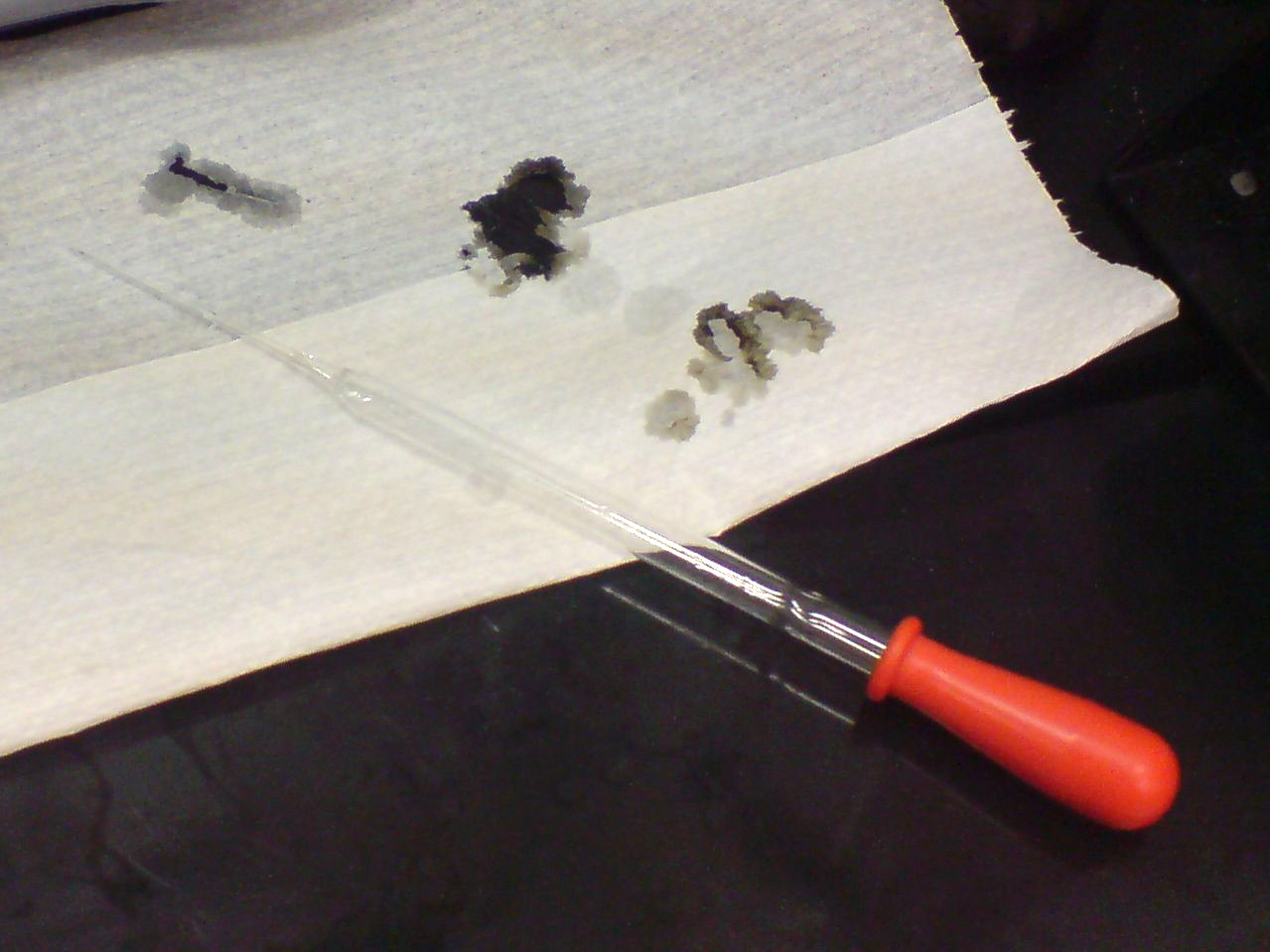

 Sulfuric acid is capable of causing very severe burns, especially when it is at high
Sulfuric acid is capable of causing very severe burns, especially when it is at high 
 Sulfuric acid must be stored carefully in containers made of nonreactive material (such as glass). Solutions equal to or stronger than 1.5 M are labeled "CORROSIVE", while solutions greater than 0.5 M but less than 1.5 M are labeled "IRRITANT". However, even the normal laboratory "dilute" grade (approximately 1 M, 10%) will char paper if left in contact for a sufficient time.
The standard first aid treatment for acid spills on the skin is, as for other
Sulfuric acid must be stored carefully in containers made of nonreactive material (such as glass). Solutions equal to or stronger than 1.5 M are labeled "CORROSIVE", while solutions greater than 0.5 M but less than 1.5 M are labeled "IRRITANT". However, even the normal laboratory "dilute" grade (approximately 1 M, 10%) will char paper if left in contact for a sufficient time.
The standard first aid treatment for acid spills on the skin is, as for other
Sulfuric acid
at ''
NIOSH Pocket Guide to Chemical HazardsCDC – Sulfuric Acid – NIOSH Workplace Safety and Health Topic
*Calculators
surface tensions
an
densities, molarities and molalities
of aqueous sulfuric acid
*Process flowsheet of sulfuric acid manufacturing b
lead chamber process
{{Authority control Acid catalysts Alchemical substances Dehydrating agents Equilibrium chemistry Hydrogen compounds Inorganic solvents Mineral acids Oxidizing acids Oxidizing agents Photographic chemicals Sulfates Sulfur oxoacids Sulfur E-number additives
American spelling
Despite the various English dialects spoken from country to country and within different regions of the same country, there are only slight regional variations in English orthography, the two most notable variations being British and America ...
and the preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Ch ...
composed of the elements sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, with the molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. It is a colorless, odorless and viscous
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
liquid that is miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also appli ...
with water.
Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance ...
and readily absorbs water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous p ...
from the air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing for ...
. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4 O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydra ...
is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boil the solution, spraying droplets of hot acid during the process. Upon contact with body tissue, sulfuric acid can cause severe acidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a s ...
chemical burn
A chemical burn occurs when living tissue is exposed to a corrosive substance (such as a strong acid, base or oxidizer) or a cytotoxic agent (such as mustard gas, lewisite or arsine). Chemical burns follow standard burn classification and may ...
s and even secondary thermal
A thermal column (or thermal) is a rising mass of buoyant air, a convective current in the atmosphere, that transfers heat energy vertically. Thermals are created by the uneven heating of Earth's surface from solar radiation, and are an example ...
burns Burns may refer to:
* Burn, an injury (plural)
People:
* Burns (surname), includes list of people and characters
Business:
* Burns London, a British guitar maker
Places:
;In the United States
* Burns, Colorado, unincorporated community in Eagle C ...
due to dehydration. Dilute sulfuric acid is substantially less hazardous without the oxidative and dehydrating properties; however, it should still be handled with care for its acidity.
Sulfuric acid is a very important commodity chemical, and a nation's sulfuric acid production is a good indicator of its industrial strength. It is widely produced with different methods, such as contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arseni ...
, wet sulfuric acid process
The wet sulfuric acid process (WSA process) is a gas desulfurization process. After Danish company Haldor Topsoe introduced this technology in 1987, it has been recognized as a process for recovering sulfur from various process gases in the form ...
, lead chamber process
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined ...
and some other methods. Sulfuric acid is also a key substance in the chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials ( oil, natural gas, air, water, metals, and minerals) into more than 70,000 different products. T ...
. It is most commonly used in fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
manufacture, but is also important in mineral processing
In the field of extractive metallurgy, mineral processing, also known as ore dressing, is the process of separating commercially valuable minerals from their ores.
History
Before the advent of heavy machinery the raw ore was broken up using ...
, oil refining, wastewater processing
Wastewater treatment is a process used to remove contaminants from wastewater and convert it into an effluent that can be returned to the water cycle. Once returned to the water cycle, the effluent creates an acceptable impact on the environmen ...
, and chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mo ...
. It has a wide range of end applications including in domestic acidic drain cleaners, as an electrolyte in lead-acid batteries, in dehydrating a compound, and in various cleaning agent
Cleaning agents or hard-surface cleaners are substances (usually liquids, powders, sprays, or granules) used to remove dirt, including dust, stains, bad smells, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removi ...
s.
Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
Physical properties
Grades of sulfuric acid
Although nearly 100% sulfuric acid solutions can be made, the subsequent loss of at the boiling point brings the concentration to 98.3% acid. The 98.3% grade is more stable in storage, and is the usual form of what is described as "concentrated sulfuric acid". Other concentrations are used for different purposes. Some common concentrations are: Please note, no EB1911 wikilink is available to this article "Chamber acid" and "tower acid" were the two concentrations of sulfuric acid produced by thelead chamber process
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined ...
, chamber acid being the acid produced in the lead chamber itself (<70% to avoid contamination with nitrosylsulfuric acid
Nitrosylsulfuric acid is the chemical compound with the formula . It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is ...
) and tower acid being the acid recovered from the bottom of the Glover tower. They are now obsolete as commercial concentrations of sulfuric acid, although they may be prepared in the laboratory from concentrated sulfuric acid if needed. In particular, "10 M" sulfuric acid (the modern equivalent of chamber acid, used in many titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant ...
s), is prepared by slowly adding 98% sulfuric acid to an equal volume of water, with good stirring: the temperature of the mixture can rise to 80 °C (176 °F) or higher.
Pure sulfuric acid
Pure sulfuric acid contains not only molecules, but is actually an equilibrium of many other chemical species, as it is shown in the table below. Pure sulfuric acid is a colorless oily liquid, and has a vapor pressure of <0.001 mmHg at 25 °C and 1 mmHg at 145.8 °C, and 98% sulfuric acid has a <1 mmHg vapor pressure at 40 °C. In the solid state, sulfuric acid is a molecular solid that formsmonoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic ...
crystals with nearly trigonal
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal ...
lattice parameters. The structure consists of layers parallel to the (010) plane, in which each molecule is connected by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
s to two others. Hydrates are known for ''n'' = 1, 2, 3, 4, 6.5, and 8, although most intermediate hydrates are stable against disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term ca ...
.
Polarity and conductivity
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achi ...
is a very polar liquid, having a dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulat ...
of around 100. It has a high electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
, caused by dissociation through protonating
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, i ...
itself, a process known as autoprotolysis
In chemistry, autoprotolysis is a chemical reaction in which a proton is transferred between two identical molecules, one of which acts as a Brønsted acid, releasing a proton which is accepted by the other molecule acting as a Brønsted base ...
.
:
The equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for autoprotolysis is
:''K''ap () (25 °C) =
The comparable equilibrium constant for water, ''K''w is 10−14, a factor of 1010 (10 billion) smaller.
In spite of the viscosity of the acid, the effective conductivities of the and ions are high due to an intramolecular proton-switch mechanism (analogous to the Grotthuss mechanism
The Grotthuss mechanism (also known as proton jumping) is the process by which an 'excess' proton or proton defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation and concomitan ...
in water), making sulfuric acid a good conductor of electricity. It is also an excellent solvent for many reactions.
Chemical properties
Reaction with water and dehydrating property
Because thehydration reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed indust ...
of sulfuric acid is highly exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
, dilution should be performed by adding the acid to the water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
rather than the water to the acid, to avoid acid splashing. Because the reaction favors the rapid protonation of water, addition of acid to the water ensures that the ''acid'' is the limiting reagent. This reaction may be thought of as the formation of hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is di ...
ions:
: ''K''a1 ≈ 103 (strong acid)
: ''K''a2 =
is the ''bisulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
'' anion and is the ''sulfate
The sulfate or sulphate ion is a polyatomic ion, polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salt (chemistry), ...
'' anion. ''K''a1 and ''K''a2 are the acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
: ...
s.
Concentrated sulfuric acid has a powerful dehydrating
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mil ...
property, removing water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
() from other chemical compounds such as table sugar
White sugar, also called table sugar, granulated sugar, or regular sugar, is a commonly used type of sugar, made either of beet sugar or cane sugar, which has undergone a refining process.
Description
The refining process completely removes ...
(sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refi ...
) and other carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ...
s, to produce carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
, steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporizat ...
, and heat. Dehydration of table sugar (sucrose) is a common laboratory demonstration. The sugar darkens as carbon is formed, and a rigid column of black, porous carbon called a carbon snake may emerge as shown in the figure.
:
Similarly, mixing starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets ...
into concentrated sulfuric acid gives elemental carbon and water that is absorbed by the sulfuric acid, slightly diluting it. The effect of this can be seen when concentrated sulfuric acid is spilled on paper, which is composed of cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
; the cellulose reacts to give a burnt appearance in which the carbon appears much like soot
Soot ( ) is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyse ...
that results from fire.
Although less dramatic, the action of the acid on cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus '' Gossypium'' in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor p ...
, even in diluted form, destroys the fabric.
:
The reaction with copper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hy ...
can also demonstrate the dehydration property of sulfuric acid. The blue crystals change into white powder as water is removed:
: (blue crystals of copper(II) sulfate pentahydrate) → (white powder of anhydrous copper(II) sulfate) +
Acid-base properties
As an acid, sulfuric acid reacts with most bases to give the corresponding sulfate. For example, the bluecopper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
salt copper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hy ...
, commonly used for electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
and as a fungicide, is prepared by the reaction of copper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It ...
with sulfuric acid:
:
Sulfuric acid can also be used to displace weaker acids from their salts. Reaction with sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
, for example, displaces acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
, , and forms sodium bisulfate
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium ...
:
:
Similarly, reacting sulfuric acid with potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and ni ...
can be used to produce nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
and a precipitate of potassium bisulfate
Potassium bisulfate/ Potassium Sulphate is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.
Preparation
More than 1 million tons were produced in 1985 as ...
. When combined with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, sulfuric acid acts both as an acid and a dehydrating agent, forming the nitronium ion
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule , or the protonation of nitric ac ...
, which is important in nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols an ...
reactions involving electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. This type of reaction, where protonation occurs on an oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
atom, is important in many organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
reactions, such as Fischer esterification
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher.
People with the surname A
* Abraham Fischer (1850–1913) South African public official
* ...
and dehydration of alcohols.
 When allowed to react with superacids, sulfuric acid can act as a base and can be protonated, forming the ion. Salts of have been prepared using the following reaction in liquid HF:
:
The above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply
When allowed to react with superacids, sulfuric acid can act as a base and can be protonated, forming the ion. Salts of have been prepared using the following reaction in liquid HF:
:
The above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric ac ...
, however, has met with failure, as pure sulfuric acid undergoes self-ionization to give ions:
:
which prevents the conversion of to by the HF/ system.
Reactions with metals
Even dilute sulfuric acid reacts with many metals via a single displacement reaction, like other typical acids, producinghydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas and salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quanti ...
s (the metal sulfate). It attacks reactive metals (metals at positions above copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
in the reactivity series
In chemistry, a reactivity series (or activity series) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. It is used to summarize information about th ...
) such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
, manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy u ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
.
:
Concentrated sulfuric acid can serve as an oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
, releasing sulfur dioxide:
:
Lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate ...
and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
, however, are resistant to sulfuric acid.
Reactions with carbon and sulfur
Hot concentrated sulfuric acid oxidizescarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
(as bituminous coal) and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
:
:
:
Reaction with sodium chloride
It reacts withsodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
, and gives hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
and sodium bisulfate
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO4. Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium ...
:
:
Electrophilic aromatic substitution
Benzene undergoeselectrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
with sulfuric acid to give the corresponding sulfonic acids:
:Occurrence
 Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric
Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of sulfur dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ...
in the presence of water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
– i.e. oxidation of sulfurous acid
Sulfurous acid (also sulfuric(IV) acid, sulphurous acid (UK), sulphuric(IV) acid (UK)) is the chemical compound with the formula . There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. ...
. When sulfur-containing fuels such as coal or oil are burned, sulfur dioxide is the main byproduct (besides the chief products carbon oxides and water).
Sulfuric acid is formed naturally by the oxidation of sulfide minerals, such as iron sulfide. The resulting water can be highly acidic and is called acid mine drainage
Acid mine drainage, acid and metalliferous drainage (AMD), or acid rock drainage (ARD) is the outflow of acidic water from metal mines or coal mines.
Acid rock drainage occurs naturally within some environments as part of the rock weathering ...
(AMD) or acid rock drainage (ARD). This acidic water is capable of dissolving metals present in sulfide ores, which results in brightly colored, toxic solutions. The oxidation of pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue giv ...
(iron sulfide) by molecular oxygen produces iron(II), or :
:
The can be further oxidized to :
:
The produced can be precipitated as the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water ...
or hydrous iron oxide
Iron(III) oxide-hydroxide or ferric oxyhydroxideA. L. Mackay (1960): "β-Ferric Oxyhydroxide". ''Mineralogical Magazine'' (''Journal of the Mineralogical Society''), volume 32, issue 250, pages 545-557. is the chemical compound of iron, oxygen ...
:
:
The iron(III) ion ("ferric iron") can also oxidize pyrite:
:
When iron(III) oxidation of pyrite occurs, the process can become rapid. pH values below zero have been measured in ARD produced by this process.
ARD can also produce sulfuric acid at a slower rate, so that the acid neutralizing capacity
Acid-neutralizing capacity or ANC in short is a measure for the overall buffering capacity against acidification of a solution, e.g. surface water or soil water.
ANC is defined as the difference between cations of strong bases and anions of ...
(ANC) of the aquifer can neutralize the produced acid. In such cases, the total dissolved solids
Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized, or micro-granular ( colloidal sol) suspended form. TDS concentrations are often repor ...
(TDS) concentration of the water can be increased from the dissolution of minerals from the acid-neutralization reaction with the minerals.
Sulfuric acid is used as a defense by certain marine species, for example, the phaeophyte alga ''Desmarestia munda'' (order Desmarestiales) concentrates sulfuric acid in cell vacuoles.
Stratospheric aerosol
In thestratosphere
The stratosphere () is the second layer of the atmosphere of the Earth, located above the troposphere and below the mesosphere. The stratosphere is an atmospheric layer composed of stratified temperature layers, with the warm layers of air ...
, the atmosphere's second layer that is generally between 10 and 50 km above Earth's surface, sulfuric acid is formed by the oxidation of volcanic sulfur dioxide by the hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
:
:
:
:
Because sulfuric acid reaches supersaturation
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liq ...
in the stratosphere, it can nucleate aerosol particles and provide a surface for aerosol growth via condensation and coagulation with other water-sulfuric acid aerosols. This results in the stratospheric aerosol layer Stratospheric sulfur aerosols are sulfur-rich particles which exist in the stratosphere region of the Earth's atmosphere. The layer of the atmosphere in which they exist is known as the Junge layer, or simply the stratospheric aerosol layer. These ...
.
Extraterrestrial sulfuric acid
The permanentVenus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never f ...
ian clouds produce a concentrated acid rain, as the clouds in the atmosphere of Earth produce water rain. Jupiter
Jupiter is the fifth planet from the Sun and the largest in the Solar System. It is a gas giant with a mass more than two and a half times that of all the other planets in the Solar System combined, but slightly less than one-thousandt ...
's moon Europa
Europa may refer to:
Places
* Europe
* Europa (Roman province), a province within the Diocese of Thrace
* Europa (Seville Metro), Seville, Spain; a station on the Seville Metro
* Europa City, Paris, France; a planned development
* Europa Cliff ...
is also thought to have an atmosphere containing sulfuric acid hydrates.
Manufacture
Sulfuric acid is produced fromsulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, oxygen and water via the conventional contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arseni ...
(DCDA) or the wet sulfuric acid process
The wet sulfuric acid process (WSA process) is a gas desulfurization process. After Danish company Haldor Topsoe introduced this technology in 1987, it has been recognized as a process for recovering sulfur from various process gases in the form ...
(WSA).
Contact process
In the first step, sulfur is burned to produce sulfur dioxide. : The sulfur dioxide is oxidized to sulfur trioxide by oxygen in the presence of avanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. This reaction is reversible and the formation of the sulfur trioxide is exothermic.
:
The sulfur trioxide is absorbed into 97–98% to form oleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid). Ol ...
(), also known as fuming sulfuric acid or pyrosulphuric acid. The oleum is then diluted with water to form concentrated sulfuric acid.
:
:
Directly dissolving in water, called the "wet sulfuric acid process
The wet sulfuric acid process (WSA process) is a gas desulfurization process. After Danish company Haldor Topsoe introduced this technology in 1987, it has been recognized as a process for recovering sulfur from various process gases in the form ...
", is rarely practiced because the reaction is extremely exothermic, resulting in a hot aerosol of sulfuric acid that requires condensation and separation.
Wet sulfuric acid process
In the first step, sulfur is burned to produce sulfur dioxide: : (−297 kJ/mol) or, alternatively,hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
() gas is incinerated to gas:
: (−1036 kJ/mol)
The sulfur dioxide then oxidized to sulfur trioxide using oxygen with vanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because ...
as catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
.
: (−198 kJ/mol) (reaction is reversible)
The sulfur trioxide is hydrated into sulfuric acid :
: (−101 kJ/mol)
The last step is the condensation of the sulfuric acid to liquid 97–98% :
: (−69 kJ/mol)
Other methods
A method that is the less well-known is the metabisulfite method, in whichmetabisulfite
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion . It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in wa ...
is placed at the bottom of a beaker and 12.6 molar concentration hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
is added. The resulting gas is bubbled through nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, which will release brown/red vapors of nitrogen dioxide as the reaction proceeds. The completion of the reaction is indicated by the ceasing of the fumes. This method does not produce an inseparable mist, which is quite convenient.
:
Burning sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
together with saltpeter (potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and ni ...
, ), in the presence of steam, has been used historically. As saltpeter decomposes, it oxidizes the sulfur to , which combines with water to produce sulfuric acid.
Alternatively, dissolving sulfur dioxide in an aqueous solution of an oxidizing metal salt such as copper(II) or iron(III) chloride:
:
:
Two less well-known laboratory methods of producing sulfuric acid, albeit in dilute form and requiring some extra effort in purification. A solution of copper(II) sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hy ...
can be electrolyzed with a copper cathode and platinum/graphite anode to give spongy copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
at cathode and evolution of oxygen gas at the anode, the solution of dilute sulfuric acid indicates completion of the reaction when it turns from blue to clear (production of hydrogen at cathode is another sign):
:
More costly, dangerous, and troublesome yet novel is the electrobromine method, which employs a mixture of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, water, and hydrobromic acid
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. ...
as the electrolytic solution. The sulfur is pushed to bottom of container under the acid solution. Then the copper cathode and platinum/graphite anode are used with the cathode near the surface and the anode is positioned at the bottom of the electrolyte to apply the current. This may take longer and emits toxic bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
/sulfur bromide vapors, but the reactant acid is recyclable. Overall, only the sulfur and water are converted to sulfuric acid and hydrogen (omitting losses of acid as vapors):
: (electrolysis of aqueous hydrogen bromide)
: (initial tribromide
Tribromide is the anion with the chemical formula Br3−, or salts containing it:
* Tetrabutylammonium tribromide
* Tetrabromophosphonium tribromide
* Pyridinium perbromide
Sodium and potassium tribromides can be prepared by reacting NaBr or ...
production, eventually reverses as depletes)
: (bromine reacts with sulfur to form disulfur dibromide)
: (oxidation and hydration of disulfur dibromide)
Prior to 1900, most sulfuric acid was manufactured by the lead chamber process
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined ...
. As late as 1940, up to 50% of sulfuric acid manufactured in the United States was produced by chamber process plants.
In the early to mid 19th century "vitriol" plants existed, among other places, in Prestonpans
Prestonpans ( gd, Baile an t-Sagairt, Scots: ''The Pans'') is a small mining town, situated approximately eight miles east of Edinburgh, Scotland, in the Council area of East Lothian. The population as of is. It is near the site of the 1745 ...
in Scotland, Shropshire
Shropshire (; alternatively Salop; abbreviated in print only as Shrops; demonym Salopian ) is a landlocked historic county in the West Midlands region of England. It is bordered by Wales to the west and the English counties of Cheshire to ...
and the Lagan Valley
The Lagan Valley (, Ulster Scots: ''Glen Lagan'') is an area of Northern Ireland between Belfast and Lisburn. The River Lagan rises on Slieve Croob in County Down and flows generally northward discharging into Belfast Lough. For a section, the ...
in County Antrim Ireland, where it was used as a bleach for linen. Early bleaching of linen was done using lactic acid from sour milk but this was a slow process and the use of vitriol sped up the bleaching process.
Uses
 Sulfuric acid is a very important commodity chemical, and indeed, a nation's sulfuric acid production is a good indicator of its industrial strength. World production in the year 2004 was about 180 million
Sulfuric acid is a very important commodity chemical, and indeed, a nation's sulfuric acid production is a good indicator of its industrial strength. World production in the year 2004 was about 180 million tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s, with the following geographic distribution: Asia 35%, North America (including Mexico) 24%, Africa 11%, Western Europe 10%, Eastern Europe and Russia 10%, Australia and Oceania 7%, South America 7%. Most of this amount (≈60%) is consumed for fertilizers, particularly superphosphates, ammonium phosphate and ammonium sulfates. About 20% is used in chemical industry for production of detergents, synthetic resins, dyestuffs, pharmaceuticals, petroleum catalysts, insecticides and antifreeze
An antifreeze is an additive which lowers the freezing point of a water-based liquid. An antifreeze mixture is used to achieve freezing-point depression for cold environments. Common antifreezes also increase the boiling point of the liquid, al ...
, as well as in various processes such as oil well acidicizing, aluminium reduction, paper sizing, and water treatment. About 6% of uses are related to pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic comp ...
s and include paints, enamels, printing inks, coated fabrics and paper, while the rest is dispersed into a multitude of applications such as production of explosives, cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria, and liquid water makes it useful for food packaging. Cellophane is highly permeable to water vapour, but may be coate ...
, acetate and viscose textiles, lubricants, non-ferrous metals, and batteries.
Industrial production of chemicals
The major use for sulfuric acid is in the "wet method" for the production ofphosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solutio ...
, used for manufacture of phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
s. In this method, phosphate rock is used, and more than 100 million tonnes are processed annually. This raw material is shown below as fluorapatite
Fluorapatite, often with the alternate spelling of fluoroapatite, is a phosphate mineral with the formula Ca5(PO4)3F (calcium fluorophosphate). Fluorapatite is a hard crystalline solid. Although samples can have various color (green, brown, b ...
, though the exact composition may vary. This is treated with 93% sulfuric acid to produce calcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, ...
, hydrogen fluoride (HF) and phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solutio ...
. The HF is removed as hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepr ...
. The overall process can be represented as:
:
Ammonium sulfate
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitroge ...
, an important nitrogen fertilizer, is most commonly produced as a byproduct from coking plants supplying the iron and steel making plants. Reacting the ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
produced in the thermal decomposition of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as stratum, rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen ...
with waste sulfuric acid allows the ammonia to be crystallized out as a salt (often brown because of iron contamination) and sold into the agro-chemicals industry.
Another important use for sulfuric acid is for the manufacture of aluminium sulfate
Aluminium sulfate is a salt with the formula Al2 (SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plant ...
, also known as paper maker's alum. This can react with small amounts of soap on paper pulp
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw mat ...
fibers to give gelatinous aluminium carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carbox ...
s, which help to coagulate the pulp fibers into a hard paper surface. It is also used for making aluminium hydroxide
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer Polymorphism (materials science), polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is Amphoter ...
, which is used at water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, inclu ...
plants to filter
Filter, filtering or filters may refer to:
Science and technology
Computing
* Filter (higher-order function), in functional programming
* Filter (software), a computer program to process a data stream
* Filter (video), a software component tha ...
out impurities, as well as to improve the taste of the water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. Aluminium sulfate
Aluminium sulfate is a salt with the formula Al2 (SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plant ...
is made by reacting bauxite
Bauxite is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (Al(OH)3), boehmite (γ-AlO(OH)) and diaspore (α-AlO(O ...
with sulfuric acid:
:
Sulfuric acid is also important in the manufacture of dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
stuffs solutions.
Sulfur–iodine cycle
Thesulfur–iodine cycle
The sulfur–iodine cycle (S–I cycle) is a three-step thermochemical cycle used to produce hydrogen.
The S–I cycle consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen. All other ch ...
is a series of thermo-chemical processes possibly usable to produce hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
from water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. It consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
.
:
The compounds of sulfur and iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
are recovered and reused, hence the consideration of the process as a cycle. This process is endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. ...
and must occur at high temperatures, so energy in the form of heat has to be supplied.
The sulfur–iodine cycle has been proposed as a way to supply hydrogen for a hydrogen-based economy. It is an alternative to electrolysis, and does not require hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s like current methods of steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen produ ...
. But note that all of the available energy in the hydrogen so produced is supplied by the heat used to make it.
The sulfur–iodine cycle is currently being researched as a feasible method of obtaining hydrogen, but the concentrated, corrosive acid at high temperatures poses currently insurmountable safety hazards if the process were built on a large scale.
Hybrid sulfur cycle
Thehybrid sulfur cycle
The hybrid sulfur cycle (HyS) is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical (inst ...
(HyS) is a two-step water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, bas ...
process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical (instead of a thermochemical) reaction for one of the two steps. The remaining thermochemical step is shared with the sulfur-iodine cycle.
Industrial cleaning agent
Sulfuric acid is used in large quantities by theiron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and steelmaking
Steelmaking is the process of producing steel from iron ore and carbon/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur and excess carbon (the most important impurity) are removed from the sourced iron, and allo ...
industry to remove
Remove, removed or remover may refer to:
* Needle remover
* Polish remover
* Staple remover
* Remove (education)
* The degree of cousinship, i.e. "once removed" or "twice removed" - see Cousin chart
See also
* Deletion (disambiguation)
* Moving ( ...
oxidation, rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), ...
, and scaling
Scaling may refer to:
Science and technology
Mathematics and physics
* Scaling (geometry), a linear transformation that enlarges or diminishes objects
* Scale invariance, a feature of objects or laws that do not change if scales of length, energ ...
from rolled sheet and billets prior to sale to the automobile
A car or automobile is a motor vehicle with wheels. Most definitions of ''cars'' say that they run primarily on roads, seat one to eight people, have four wheels, and mainly transport people instead of goods.
The year 1886 is regarded ...
and major appliances
A major appliance, also known as a large domestic appliance or large electric appliance or simply a large appliance, large domestic, or large electric, is a non-portable or semi-portable machine used for routine housekeeping tasks such as cook ...
industry. Used acid is often recycled using a spent acid regeneration (SAR) plant. These plants combust spent acid with natural gas, refinery gas, fuel oil or other fuel sources. This combustion process produces gaseous sulfur dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic ...
() and sulfur trioxide () which are then used to manufacture "new" sulfuric acid. SAR plants are common additions to metal smelting plants, oil refineries, and other industries where sulfuric acid is consumed in bulk, as operating a SAR plant is much cheaper than the recurring costs of spent acid disposal and new acid purchases.
Hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3% ...
() can be added to sulfuric acid to produce piranha solution
Piranha solution, also known as piranha etch, is a mixture of sulfuric acid () and hydrogen peroxide (), used to clean organic residues off substrates. Because the mixture is a strong oxidizing agent, it will decompose most organic matter, and ...
, a powerful but very toxic cleaning solution with which substrate surfaces can be cleaned. Piranha solution is typically used in the microelectronics industry, and also in laboratory settings to clean glassware.
Catalyst
Sulfuric acid is used for a variety of other purposes in the chemical industry. For example, it is the usual acid catalyst for the conversion of cyclohexanone oxime tocaprolactam
Caprolactam (CPL) is an organic compound with the formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast majority is us ...
, used for making nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pet ...
. It is used for making hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
from salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quanti ...
via the Mannheim process. Much is used in petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
refining, for example as a catalyst for the reaction of isobutane with isobutylene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Producti ...
to give isooctane
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale ...
, a compound that raises the octane rating of gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic ...
(petrol). Sulfuric acid is also often used as a dehydrating or oxidizing agent in industrial reactions, such as the dehydration of various sugars to form solid carbon.
Electrolyte
 Sulfuric acid acts as the electrolyte in lead–acid batteries (lead-acid accumulator):
At
Sulfuric acid acts as the electrolyte in lead–acid batteries (lead-acid accumulator):
At anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemoni ...
:
:
At cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction i ...
:
:
Domestic uses
Sulfuric acid at high concentrations is frequently the major ingredient in acidic drain cleaners which are used to remove grease, hair,tissue paper
Tissue paper or simply tissue is a lightweight paper or, light crêpe paper. Tissue can be made from recycled paper pulp on a paper machine.
Tissue paper is very versatile, and different kinds of tissue are made to best serve these purposes, whi ...
, etc. Similar to their alkaline versions, such drain openers can dissolve fats and proteins via hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
. Moreover, as concentrated sulfuric acid has a strong dehydrating property, it can remove tissue paper via dehydrating process as well. Since the acid may react with water vigorously, such acidic drain openers should be added slowly into the pipe to be cleaned.
History
 The study of
The study of vitriol
Vitriol is the general chemical name encompassing a class of chemical compound comprising sulfates of certain metalsoriginally, iron or copper. Those mineral substances were distinguished by their color, such as green vitriol for hydrated iron(II ...
, a category of glassy minerals from which the acid can be derived, began in ancient times
Ancient history is a time period from the beginning of writing and recorded human history to as far as late antiquity. The span of recorded history is roughly 5,000 years, beginning with the Sumerian cuneiform script. Ancient history cove ...
. Sumerians had a list of types of vitriol that they classified according to the substances' color. Some of the earliest discussions on the origin and properties of vitriol is in the works of the Greek physician Dioscorides
Pedanius Dioscorides ( grc-gre, Πεδάνιος Διοσκουρίδης, ; 40–90 AD), “the father of pharmacognosy”, was a Greek physician, pharmacologist, botanist, and author of '' De materia medica'' (, On Medical Material) —a 5-vo ...
(first century AD) and the Roman naturalist Pliny the Elder
Gaius Plinius Secundus (AD 23/2479), called Pliny the Elder (), was a Roman author, naturalist and natural philosopher, and naval and army commander of the early Roman Empire, and a friend of the emperor Vespasian. He wrote the encyclopedic ...
(23–79 AD). Galen
Aelius Galenus or Claudius Galenus ( el, Κλαύδιος Γαληνός; September 129 – c. AD 216), often Anglicized as Galen () or Galen of Pergamon, was a Greek physician, surgeon and philosopher in the Roman Empire. Considered to be on ...
also discussed its medical use. Metallurgical uses for vitriolic substances were recorded in the Hellenistic alchemical works of Zosimos of Panopolis
Zosimos of Panopolis ( el, Ζώσιμος ὁ Πανοπολίτης; also known by the Latin name Zosimus Alchemista, i.e. "Zosimus the Alchemist") was a Greco-Egyptian alchemist and Gnostic mystic who lived at the end of the 3rd and beginning ...
, in the treatise ''Phisica et Mystica'', and the Leyden papyrus X
The Leyden papyrus X (P. Leyden X) is a papyrus codex written in Greek at about the end of the 3rd century A.D.E.R.Caley, ''The Leyden Paprus X: An English Translation with Brief Notes''p.1149 "These two papyri have, however, upon the basis of un ...
.
Medieval Islamic chemists like Jābir ibn Ḥayyān (died c. 806 – c. 816 AD, known in Latin as Geber), Abū Bakr al-Rāzī
Abū Bakr al-Rāzī (full name: ar, أبو بکر محمد بن زکریاء الرازي, translit=Abū Bakr Muḥammad ibn Zakariyyāʾ al-Rāzī, label=none), () rather than ar, زکریاء, label=none (), as for example in , or in . In m ...
(865 – 925 AD, known in Latin as Rhazes), Ibn Sina
Ibn Sina ( fa, ابن سینا; 980 – June 1037 CE), commonly known in the West as Avicenna (), was a Persian polymath who is regarded as one of the most significant physicians, astronomers, philosophers, and writers of the Islamic G ...
(980 – 1037 AD, known in Latin as Avicenna), and Muḥammad ibn Ibrāhīm al-Watwat
Muhammad ( ar, مُحَمَّد; 570 – 8 June 632 CE) was an Arab religious, social, and political leader and the founder of Islam. According to Islamic doctrine, he was a prophet divinely inspired to preach and confirm the monoth ...
(1234 – 1318 AD) included vitriol in their mineral classification lists.
Sulfuric acid was called "oil of vitriol" by medieval European alchemists because it was prepared by roasting "green vitriol" (iron(II) sulfate
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·''x''H2O. These compounds exist most commonly as the hepta hydrate (''x'' = 7) but several values for x are kno ...
) in an iron retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heated. Th ...
. The first vague allusions to it appear in the works of Vincent of Beauvais
Vincent of Beauvais ( la, Vincentius Bellovacensis or ''Vincentius Burgundus''; c. 1264) was a Dominican friar at the Cistercian monastery of Royaumont Abbey, France. He is known mostly for his '' Speculum Maius'' (''Great mirror''), a major wor ...
, in the ''Compositum de Compositis'' ascribed to Saint Albertus Magnus
Albertus Magnus (c. 1200 – 15 November 1280), also known as Saint Albert the Great or Albert of Cologne, was a German Dominican friar, philosopher, scientist, and bishop. Later canonised as a Catholic saint, he was known during his li ...
, and in pseudo-Geber
Pseudo-Geber (or "Latin pseudo-Geber") is the presumed author or group of authors responsible for a corpus of pseudepigraphic alchemical writings dating to the late 13th and early 14th centuries. These writings were falsely attributed to Jabir ...
's ''Summa perfectionis'' (all thirteenth century AD).
In the seventeenth century, the German-Dutch chemist Johann Glauber
Johann Rudolf Glauber (10 March 1604 – 16 March 1670) was a German-Dutch alchemist and chemist. Some historians of science have described him as one of the first chemical engineers. His discovery of sodium sulfate in 1625 led to the compou ...
prepared sulfuric acid by burning sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
together with saltpeter (potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and ni ...
, ), in the presence of steam. As saltpeter decomposes, it oxidizes the sulfur to , which combines with water to produce sulfuric acid. In 1736, Joshua Ward, a London pharmacist, used this method to begin the first large-scale production of sulfuric acid.
In 1746 in Birmingham, John Roebuck
John Roebuck of Kinneil FRS FRSE (1718 – 17 July 1794) was an English inventor and industrialist who played an important role in the Industrial Revolution and who is known for developing the industrial-scale manufacture of sulphuric ac ...
adapted this method to produce sulfuric acid in lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate ...
-lined chambers, which were stronger, less expensive, and could be made larger than the previously used glass containers. This process allowed the effective industrialization of sulfuric acid production. After several refinements, this method, called the lead chamber process
The lead chamber process was an industrial method used to produce sulfuric acid in large quantities. It has been largely supplanted by the contact process.
In 1746 in Birmingham, England, John Roebuck began producing sulfuric acid in lead-lined ...
or "chamber process", remained the standard for sulfuric acid production for almost two centuries.
Sulfuric acid created by John Roebuck's process approached a 65% concentration. Later refinements to the lead chamber process by French chemist Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
and British chemist John Glover improved concentration to 78%. However, the manufacture of some dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
s and other chemical processes require a more concentrated product. Throughout the 18th century, this could only be made by dry distilling minerals in a technique similar to the original alchemical
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim wor ...
processes. Pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue giv ...
(iron disulfide, ) was heated in air to yield iron(II) sulfate, , which was oxidized by further heating in air to form iron(III) sulfate
Iron(III) sulfate (or ferric sulfate), is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulfate". Solutions are used in dyeing as a mor ...
, , which, when heated to 480 °C, decomposed to iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally ...
and sulfur trioxide, which could be passed through water to yield sulfuric acid in any concentration. However, the expense of this process prevented the large-scale use of concentrated sulfuric acid.
In 1831, British vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to ...
merchant Peregrine Phillips patented the contact process
The contact process is the current method of producing sulfuric acid in the high concentrations needed for industrial processes. Platinum was originally used as the catalyst for this reaction; however, as it is susceptible to reacting with arseni ...
, which was a far more economical process for producing sulfur trioxide and concentrated sulfuric acid. Today, nearly all of the world's sulfuric acid is produced using this method.
Safety
Laboratory hazards


 Sulfuric acid is capable of causing very severe burns, especially when it is at high
Sulfuric acid is capable of causing very severe burns, especially when it is at high concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
s. In common with other corrosive acids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
and alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
, it readily decomposes proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respondi ...
and lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids includ ...
through amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
and ester hydrolysis
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
upon contact with living tissues
In biology, tissue is a biological organizational level between cells and a complete organ. A tissue is an ensemble of similar cells and their extracellular matrix from the same origin that together carry out a specific function. Organs are th ...
, such as skin
Skin is the layer of usually soft, flexible outer tissue covering the body of a vertebrate animal, with three main functions: protection, regulation, and sensation.
Other cuticle, animal coverings, such as the arthropod exoskeleton, have diffe ...
and flesh
Flesh is any aggregation of soft tissues of an organism. Various multicellular organisms have soft tissues that may be called "flesh". In mammals, including humans, ''flesh'' encompasses muscles, fats and other loose connective tissues, but ...
. In addition, it exhibits a strong dehydrating property on carbohydrates
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may ...
, liberating extra heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
and causing secondary thermal burns. Accordingly, it rapidly attacks the cornea
The cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Along with the anterior chamber and lens, the cornea refracts light, accounting for approximately two-thirds of the eye's total optical ...
and can induce permanent blindness if splashed onto eye
Eyes are organs of the visual system. They provide living organisms with vision, the ability to receive and process visual detail, as well as enabling several photo response functions that are independent of vision. Eyes detect light and conv ...
s. If ingested, it damages internal organs
In biology, an organ is a collection of tissues joined in a structural unit to serve a common function. In the hierarchy of life, an organ lies between tissue and an organ system. Tissues are formed from same type cells to act together in a ...
irreversibly and may even be fatal. Protective equipment
Personal protective equipment (PPE) is protective clothing, helmets, goggles, or other garments or equipment designed to protect the wearer's body from injury or infection. The hazards addressed by protective equipment include physical, elec ...
should hence always be used when handling it. Moreover, its strong oxidizing property makes it highly corrosive to many metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s and may extend its destruction on other materials. Because of such reasons, damage posed by sulfuric acid is potentially more severe than that by other comparable strong acids
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutio ...
, such as hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
and nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
.

corrosive agents
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Etymology
The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', ...
, irrigation with large quantities of water. Washing is continued for at least ten to fifteen minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed immediately and the underlying skin washed thoroughly.
Dilution hazards
Preparation of the diluted acid can be dangerous due to the heat released in the dilution process. To avoid splattering, the concentrated acid is usually added to water and not the other way around. A saying used to remember this is "Do like you oughta, add the acid to the water". Water has a higher heat capacity than the acid, and so a vessel of cold water will absorb heat as acid is added. Also, because the acid is denser than water, it sinks to the bottom. Heat is generated at the interface between acid and water, which is at the bottom of the vessel. Acid will not boil, because of its higher boiling point. Warm water near the interface rises due toconvection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the c ...
, which cools the interface, and prevents boiling of either acid or water.
In contrast, addition of water to concentrated sulfuric acid results in a thin layer of water on top of the acid. Heat generated in this thin layer of water can boil, leading to the dispersal of a sulfuric acid aerosol
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of ant ...
or worse, an explosion
An explosion is a rapid expansion in volume associated with an extreme outward release of energy, usually with the generation of high temperatures and release of high-pressure gases. Supersonic explosions created by high explosives are kno ...
.
Preparation of solutions greater than 6 M (35%) in concentration is most dangerous, because the heat produced may be sufficient to boil the diluted acid: efficient mechanical stirring and external cooling (such as an ice bath) are essential.
Reaction rates double for about every 10-degree Celsius increase in temperature. Therefore, the reaction will become more violent as dilution proceeds, unless the mixture is given time to cool. Adding acid to warm water will cause a violent reaction.
On a laboratory scale, sulfuric acid can be diluted by pouring concentrated acid onto crushed ice made from de-ionized water. The ice melts in an endothermic process while dissolving the acid. The amount of heat needed to melt the ice in this process is greater than the amount of heat evolved by dissolving the acid so the solution remains cold. After all the ice has melted, further dilution can take place using water.
Industrial hazards
Sulfuric acid is non-flammable. The main occupational risks posed by this acid are skin contact leading to burns (see above) and the inhalation of aerosols. Exposure to aerosols at high concentrations leads to immediate and severe irritation of the eyes, respiratory tract and mucous membranes: this ceases rapidly after exposure, although there is a risk of subsequentpulmonary edema
Pulmonary edema, also known as pulmonary congestion, is excessive liquid accumulation in the tissue and air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia and respiratory failure. It is du ...
if tissue damage has been more severe. At lower concentrations, the most commonly reported symptom of chronic exposure to sulfuric acid aerosols is erosion of the teeth, found in virtually all studies: indications of possible chronic damage to the respiratory tract
The respiratory tract is the subdivision of the respiratory system involved with the process of respiration in mammals. The respiratory tract is lined with respiratory epithelium as respiratory mucosa.
Air is breathed in through the nose to ...
are inconclusive as of 1997. Repeated occupational exposure to sulfuric acid mists may increase the chance of lung cancer by up to 64 percent. In the United States, the permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits are established by the Occupational ...
(PEL) for sulfuric acid is fixed at 1 mg/m3: limits in other countries are similar. There have been reports of sulfuric acid ingestion leading to vitamin B12 deficiency
Vitamin B12 deficiency, also known as cobalamin deficiency, is the medical condition in which the blood and tissue have a lower than normal level of vitamin B12. Symptoms can vary from none to severe. Mild deficiency may have few or absent symp ...
with subacute combined degeneration. The spinal cord is most often affected in such cases, but the optic nerves may show demyelination
A demyelinating disease is any disease of the nervous system in which the myelin sheath of neurons is damaged. This damage impairs the conduction of signals in the affected nerves. In turn, the reduction in conduction ability causes deficiency i ...
, loss of axon
An axon (from Greek ἄξων ''áxōn'', axis), or nerve fiber (or nerve fibre: see spelling differences), is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action ...
s and gliosis
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, mi ...
.
Legal restrictions
International commerce of sulfuric acid is controlled under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, 1988, which lists sulfuric acid under Table II of the convention as a chemical frequently used in the illicit manufacture of narcotic drugs or psychotropic substances.See also
* Aqua regia *Diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
– also known as "sweet oil of vitriol"
*Piranha solution
Piranha solution, also known as piranha etch, is a mixture of sulfuric acid () and hydrogen peroxide (), used to clean organic residues off substrates. Because the mixture is a strong oxidizing agent, it will decompose most organic matter, and ...
*Sulfur oxoacid
Sulfur oxoacids are chemical compounds that contain sulfur, oxygen, and hydrogen. The best known and most important industrially used is sulfuric acid. Sulfur has several oxoacids; however, some of these are known only from their salts (these are ...
* Sulfuric acid poisoning
References
External links
*Sulfuric acid
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics ...
'' (University of Nottingham)NIOSH Pocket Guide to Chemical Hazards
*Calculators
surface tensions
an
densities, molarities and molalities
of aqueous sulfuric acid
*Process flowsheet of sulfuric acid manufacturing b
lead chamber process
{{Authority control Acid catalysts Alchemical substances Dehydrating agents Equilibrium chemistry Hydrogen compounds Inorganic solvents Mineral acids Oxidizing acids Oxidizing agents Photographic chemicals Sulfates Sulfur oxoacids Sulfur E-number additives