
X-ray crystallography is the experimental science determining the atomic and molecular structure of a
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macr ...
, in which the crystalline structure causes a beam of incident
X-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
s to
diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a
crystallographer can produce a three-dimensional picture of the density of
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
s within the crystal. From this
electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial ...
, the mean positions of the atoms in the crystal can be determined, as well as their
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s, their
crystallographic disorder, and various other information.
Since many materials can form crystals—such as
salts,
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s,
mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. ...
s,
semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
s, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various materials, especially minerals and
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s. The method also revealed the structure and function of many biological molecules, including
vitamin
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an essential micronutrient that an organism needs in small quantities for the proper functioning of its metabolism. Essential nut ...
s, drugs,
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s and
nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main ...
s such as
DNA. X-ray crystallography is still the primary method for characterizing the atomic structure of new materials and in discerning materials that appear similar by other
experiment
An experiment is a procedure carried out to support or refute a hypothesis, or determine the efficacy or likelihood of something previously untried. Experiments provide insight into cause-and-effect by demonstrating what outcome occurs wh ...
s. X-ray
crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ...
s can also account for unusual
electronic or
elastic properties of a material, shed light on chemical interactions and processes, or serve as the basis for
designing pharmaceuticals against diseases.
In a single-crystal X-ray diffraction measurement, a crystal is mounted on a
goniometer. The goniometer is used to position the crystal at selected orientations. The crystal is illuminated with a finely focused
monochromatic
A monochrome or monochromatic image, object or palette is composed of one color (or values of one color). Images using only shades of grey are called grayscale (typically digital) or black-and-white (typically analog). In physics, monochro ...
beam of X-rays, producing a
diffraction pattern of regularly spaced spots known as ''reflections''. The two-dimensional images taken at different orientations are converted into a three-dimensional model of the density of electrons within the crystal using the mathematical method of
Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
s, combined with chemical data known for the sample. Poor resolution (fuzziness) or even errors may result if the crystals are too small, or not uniform enough in their internal makeup.
X-ray crystallography is related to several other methods for determining atomic structures. Similar diffraction patterns can be produced by scattering electrons or
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s, which are likewise interpreted by
Fourier transformation. If single crystals of sufficient size cannot be obtained, various other X-ray methods can be applied to obtain less detailed information; such methods include
fiber diffraction,
powder diffraction and (if the sample is not crystallized)
small-angle X-ray scattering (SAXS).
If the material under investigation is only available in the form of
nanocrystalline
A nanocrystalline (NC) material is a polycrystalline material with a crystallite size of only a few nanometers. These materials fill the gap between amorphous materials without any long range order and conventional coarse-grained materials. Def ...
powders or suffers from poor crystallinity, the methods of
electron crystallography can be applied for determining the atomic structure.
For all above mentioned X-ray diffraction methods, the scattering is
elastic; the scattered X-rays have the same
wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
as the incoming X-ray. By contrast, ''inelastic'' X-ray scattering methods are useful in studying excitations of the sample such as
plasmon
In physics, a plasmon is a quantum of plasma oscillation. Just as light (an optical oscillation) consists of photons, the plasma oscillation consists of plasmons. The plasmon can be considered as a quasiparticle since it arises from the qua ...
s, crystal-field and orbital excitations,
magnons, and
phonon
In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle, a phonon is an excited state in the quantum mechanical ...
s, rather than the distribution of its atoms.
History
Early scientific history of crystals and X-rays

Crystals, though long admired for their regularity and symmetry, were not investigated scientifically until the 17th century.
Johannes Kepler hypothesized in his work ''Strena seu de Nive Sexangula'' (A New Year's Gift of Hexagonal Snow) (1611) that the hexagonal symmetry of
snowflake crystals was due to a regular packing of spherical water particles.

The Danish scientist
Nicolas Steno (1669) pioneered experimental investigations of crystal symmetry. Steno showed that the angles between the faces are the same in every exemplar of a particular type of crystal, and
René Just Haüy (1784) discovered that every face of a crystal can be described by simple stacking patterns of blocks of the same shape and size. Hence,
William Hallowes Miller
Prof William Hallowes Miller FRS HFRSE LLD DCL (6 April 180120 May 1880) was a Welsh mineralogist and laid the foundations of modern crystallography.
Miller indices are named after him, the method having been described in his ''Treatise on Cr ...
in 1839 was able to give each face a unique label of three small integers, the
Miller indices
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and ''� ...
which remain in use today for identifying crystal faces. Haüy's study led to the correct idea that crystals are a regular three-dimensional array (a
Bravais lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
) of atoms and
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s; a single
unit cell is repeated indefinitely along three principal directions that are not necessarily perpendicular. In the 19th century, a complete catalog of the possible symmetries of a crystal was worked out by
Johan Hessel,
Auguste Bravais,
Evgraf Fedorov,
Arthur Schönflies
Arthur Moritz Schoenflies (; 17 April 1853 – 27 May 1928), sometimes written as Schönflies, was a German mathematician, known for his contributions to the application of group theory to crystallography, and for work in topology.
Schoenflies ...
and (belatedly)
William Barlow William Barlow may refer to:
Religious figures
*William Barlow (bishop of Chichester) (c. 1498–1568), English cleric
* William Barlow (bishop of Lincoln) (died 1613), Anglican priest and courtier, served as Bishop of Rochester and Bishop of Linco ...
(1894). From the available data and physical reasoning, Barlow proposed several crystal structures in the 1880s that were validated later by X-ray crystallography; however, the available data were too scarce in the 1880s to accept his models as conclusive.
 Wilhelm Röntgen
Wilhelm Röntgen discovered X-rays in 1895, just as the studies of crystal symmetry were being completed.
Physicists were uncertain of the nature of X-rays, but soon suspected that they were waves of
electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible ...
, a form of
light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
. The
Maxwell
Maxwell may refer to:
People
* Maxwell (surname), including a list of people and fictional characters with the name
** James Clerk Maxwell, mathematician and physicist
* Justice Maxwell (disambiguation)
* Maxwell baronets, in the Baronetage o ...
theory of
electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible ...
was well accepted among scientists, and experiments by
Charles Glover Barkla showed that X-rays exhibited phenomena associated with electromagnetic waves, including transverse
polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
and
spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to iden ...
s akin to those observed in the visible wavelengths. Barkla created the x-ray notation, as well, noting in 1909 two separate types of diffraction beams, at first, naming them "A" and "B" and then supposing that there may be lines prior to "A", he started an alphabet numbering beginning with "K."
[Michael Eckert, Disputed discovery: the beginnings of X-ray diffraction in crystals in 1912 and its repercussions, January 2011, Acta crystallographica. Section A, Foundations of crystallography 68(1):30-39 This Laue centennial article has also been published in Zeitschrift für Kristallographie ckert (2012). Z. Kristallogr. 227 , 27–35] Single-slit experiments in the laboratory of
Arnold Sommerfeld suggested that X-rays had a
wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
of about 1
angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
. X-rays are not only waves but are also
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are Massless particle, massless ...
s, and have particle properties causing Sommerfeld to coin the name, Bremsstrahlung, for this wavelike type of diffraction.
Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theor ...
introduced the photon concept in 1905, but it was not broadly accepted until 1922, when
Arthur Compton confirmed it by the scattering of X-rays from electrons. The particle-like properties of X-rays, such as their ionization of gases, had prompted
William Henry Bragg to argue in 1907 that X-rays were ''not'' electromagnetic radiation. Bragg's view proved unpopular and the observation of
X-ray diffraction by
Max von Laue in 1912
confirmed for most scientists that X-rays are a form of electromagnetic radiation.
X-ray diffraction

Crystals are regular arrays of atoms, and X-rays can be considered waves of electromagnetic radiation. Atoms scatter X-ray waves, primarily through the atoms' electrons. Just as an ocean wave striking a lighthouse produces secondary circular waves emanating from the lighthouse, so an X-ray striking an electron produces secondary spherical waves emanating from the electron. This phenomenon is known as
elastic scattering, and the electron (or lighthouse) is known as the ''scatterer''. A regular array of scatterers produces a regular array of spherical waves. Although these waves cancel one another out in most directions through
destructive interference, they add constructively in a few specific directions, determined by
Bragg's law:
:
Here ''d'' is the spacing between diffracting planes,
is the incident angle, ''n'' is any integer, and λ is the wavelength of the beam. These specific directions appear as spots on the
diffraction pattern called ''reflections''. Thus, X-ray diffraction results from an electromagnetic wave (the X-ray) impinging on a regular array of scatterers (the repeating arrangement of atoms within the crystal).
X-rays are used to produce the diffraction pattern because their wavelength λ is typically the same order of magnitude (1–100 angstroms) as the spacing ''d'' between planes in the crystal. In principle, any wave impinging on a regular array of scatterers produces
diffraction, as predicted first by
Francesco Maria Grimaldi in 1665. To produce significant diffraction, the spacing between the scatterers and the wavelength of the impinging wave should be similar in size. For illustration, the diffraction of sunlight through a bird's feather was first reported by
James Gregory in the later 17th century. The first artificial
diffraction gratings for visible light were constructed by
David Rittenhouse in 1787, and
Joseph von Fraunhofer
Joseph Ritter von Fraunhofer (; ; 6 March 1787 – 7 June 1826) was a German physicist and optical lens manufacturer. He made optical glass, an achromatic telescope, and objective lenses. He also invented the spectroscope and developed diffr ...
in 1821. However, visible light has too long a wavelength (typically, 5500 angstroms) to observe diffraction from crystals. Prior to the first X-ray diffraction experiments, the spacings between lattice planes in a crystal were not known with certainty.
The idea that crystals could be used as a
diffraction grating for
X-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
s arose in 1912 in a conversation between
Paul Peter Ewald
Paul Peter Ewald, FRS (January 23, 1888 in Berlin, Germany – August 22, 1985 in Ithaca, New York) was a German crystallographer and physicist, a pioneer of X-ray diffraction methods.
Education
Ewald received his early education in the class ...
and
Max von Laue in the
English Garden in
Munich
Munich ( ; german: München ; bar, Minga ) is the capital and most populous city of the German state of Bavaria. With a population of 1,558,395 inhabitants as of 31 July 2020, it is the third-largest city in Germany, after Berlin and Ha ...
. Ewald had proposed a resonator model of crystals for his thesis, but this model could not be validated using
visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 tera ...
, since the wavelength was much larger than the spacing between the resonators. Von Laue realized that electromagnetic radiation of a shorter wavelength was needed to observe such small spacings, and suggested that X-rays might have a wavelength comparable to the unit-cell spacing in crystals. Von Laue worked with two technicians, Walter Friedrich and his assistant Paul Knipping, to shine a beam of X-rays through a
copper sulfate crystal and record its diffraction on a
photographic plate. After being developed, the plate showed a large number of well-defined spots arranged in a pattern of intersecting circles around the spot produced by the central beam.
Von Laue developed a law that connects the scattering angles and the size and orientation of the unit-cell spacings in the crystal, for which he was awarded the
Nobel Prize in Physics
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then " ...
in 1914.
Scattering
As described in the
mathematical derivation below, the X-ray scattering is determined by the density of electrons within the crystal. Since the energy of an X-ray is much greater than that of a valence electron, the scattering may be modeled as
Thomson scattering, the interaction of an electromagnetic ray with a free electron. This model is generally adopted to describe the polarization of the scattered radiation.
The intensity of
Thomson scattering for one particle with mass ''m'' and elementary charge ''q'' is:
:
Hence the atomic nuclei, which are much heavier than an electron, contribute negligibly to the scattered X-rays. Consequently, the coherent scattering detected from an atom can be accurately approximated by analyzing the collective scattering from the electrons in the system.
Development from 1912 to 1920
After Von Laue's pioneering research, the field developed rapidly, most notably by physicists
William Lawrence Bragg and his father
William Henry Bragg. In 1912–1913, the younger Bragg developed
Bragg's law, which connects the observed scattering with reflections from evenly spaced planes within the crystal.
The Braggs, father and son, shared the 1915 Nobel Prize in Physics for their work in crystallography. The earliest structures were generally simple and marked by one-dimensional symmetry. However, as computational and experimental methods improved over the next decades, it became feasible to deduce reliable atomic positions for more complicated two- and three-dimensional arrangements of atoms in the unit-cell.
The potential of X-ray crystallography for determining the structure of molecules and minerals—then only known vaguely from chemical and hydrodynamic experiments—was realized immediately. The earliest structures were simple inorganic crystals and minerals, but even these revealed fundamental laws of physics and chemistry. The first atomic-resolution structure to be "solved" (i.e., determined) in 1914 was that of
table salt. The distribution of electrons in the table-salt structure showed that crystals are not necessarily composed of
covalently bonded molecules, and proved the existence of
ionic compounds. The structure of
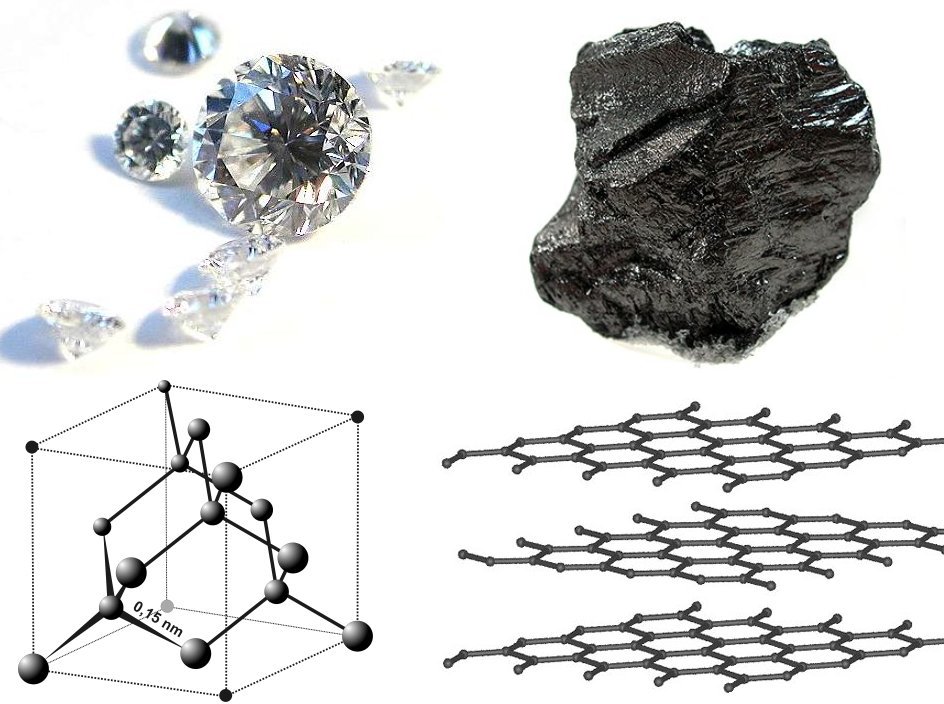
diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, ...
was solved in the same year,
proving the tetrahedral arrangement of its chemical bonds and showing that the length of C–C single bond was 1.52 angstroms. Other early structures included
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
,
calcium fluoride (CaF
2, also known as ''fluorite''),
calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scra ...
(CaCO
3) and
pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue giv ...
(FeS
2)
in 1914;
spinel
Spinel () is the magnesium/aluminium member of the larger spinel group of minerals. It has the formula in the cubic crystal system. Its name comes from the Latin word , which means ''spine'' in reference to its pointed crystals.
Properties
...
(MgAl
2O
4) in 1915; the
rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wa ...
and
anatase
Anatase is a metastable mineral form of titanium dioxide (TiO2) with a tetragonal crystal structure. Although colorless or white when pure, anatase in nature is usually a black solid due to impurities. Three other polymorphs (or mineral forms ...
forms of
titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble ...
(TiO
2) in 1916;
pyrochroite Mn(OH)
2 and, by extension,
brucite Mg(OH)
2 in 1919. Also in 1919,
sodium nitrate (NaNO
3) and caesium dichloroiodide (CsICl
2) were determined by
Ralph Walter Graystone Wyckoff, and the
wurtzite (hexagonal ZnS) structure became known in 1920.
The structure of
graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
was solved in 1916 by the related method of
powder diffraction, which was developed by
Peter Debye
Peter Joseph William Debye (; ; March 24, 1884 – November 2, 1966) was a Dutch-American physicist and physical chemistry, physical chemist, and List of Nobel laureates in Chemistry, Nobel laureate in Chemistry.
Biography
Early life
Born Petr ...
and
Paul Scherrer and, independently, by
Albert Hull in 1917. The structure of graphite was determined from single-crystal diffraction in 1924 by two groups independently. Hull also used the powder method to determine the structures of various metals, such as iron and magnesium.
Cultural and aesthetic importance
In 1951, the Festival Pattern Group at the
Festival of Britain hosted a collaborative group of textile manufacturers and experienced crystallographers to design lace and prints based on the X-ray crystallography of
insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabol ...
,
china clay, and
hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
. One of the leading scientists of the project was Dr.
Helen Megaw (1907–2002), the Assistant Director of Research at the Cavendish Laboratory in Cambridge at the time. Megaw is credited as one of the central figures who took inspiration from crystal diagrams and saw their potential in design.
In 2008, the Wellcome Collection in London curated an exhibition on the Festival Pattern Group called "From Atom to Patterns".
Contributions to chemistry and material science
X-ray crystallography has led to a better understanding of
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s and
non-covalent interactions. The initial studies revealed the typical radii of atoms, and confirmed many theoretical models of chemical bonding, such as the tetrahedral bonding of carbon in the diamond structure,
the octahedral bonding of metals observed in ammonium hexachloroplatinate (IV), and the resonance observed in the planar carbonate group
and in aromatic molecules.
's 1928 structure of
hexamethylbenzene established the hexagonal symmetry of
benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
and showed a clear difference in bond length between the aliphatic C–C bonds and aromatic C–C bonds; this finding led to the idea of
resonance between chemical bonds, which had profound consequences for the development of chemistry. Her conclusions were anticipated by
William Henry Bragg, who published models of
naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromat ...
and
anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is ...
in 1921 based on other molecules, an early form of
molecular replacement.
Also in the 1920s,
Victor Moritz Goldschmidt and later
Linus Pauling developed rules for eliminating chemically unlikely structures and for determining the relative sizes of atoms. These rules led to the structure of
brookite (1928) and an understanding of the relative stability of the
rutile
Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wa ...
,
brookite and
anatase
Anatase is a metastable mineral form of titanium dioxide (TiO2) with a tetragonal crystal structure. Although colorless or white when pure, anatase in nature is usually a black solid due to impurities. Three other polymorphs (or mineral forms ...
forms of
titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble ...
.
The distance between two bonded atoms is a sensitive measure of the bond strength and its
bond order; thus, X-ray crystallographic studies have led to the discovery of even more exotic types of bonding in
inorganic chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disc ...
, such as metal-metal double bonds, metal-metal quadruple bonds, and three-center, two-electron bonds. X-ray crystallography—or, strictly speaking, an inelastic
Compton scattering experiment—has also provided evidence for the partly covalent character of
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
s. In the field of
organometallic chemistry, the X-ray structure of
ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, ...
initiated scientific studies of
sandwich compounds, while that of
Zeise's salt stimulated research into "back bonding" and metal-pi complexes. Finally, X-ray crystallography had a pioneering role in the development of
supramolecular chemistry, particularly in clarifying the structures of the
crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . I ...
s and the principles of
host–guest chemistry.
X-ray diffraction is a very powerful tool in
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
development. Ex-situ measurements are carried out routinely for checking the crystal structure of materials or to unravel new structures. In-situ experiments give comprehensive understanding about the structural stability of catalysts under reaction conditions.
In material sciences, many complicated
inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemis ...
and
organometallic systems have been analyzed using single-crystal methods, such as
fullerenes,
metalloporphyrins, and other complicated compounds. Single-crystal diffraction is also used in the
pharmaceutical industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, Vaccine, vaccinate them, or alleviate s ...
, due to recent problems with
polymorphs. The major factors affecting the quality of single-crystal structures are the crystal's size and regularity;
recrystallization is a commonly used technique to improve these factors in small-molecule crystals. The
Cambridge Structural Database contains over 1,000,000 structures as of June 2019; over 99% of these structures were determined by X-ray diffraction.
Mineralogy and metallurgy

Since the 1920s, X-ray diffraction has been the principal method for determining the arrangement of atoms in minerals and
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s. The application of X-ray crystallography to
mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the proce ...
began with the structure of
garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different ...
, which was determined in 1924 by Menzer. A systematic X-ray crystallographic study of the
silicates was undertaken in the 1920s. This study showed that, as the
Si/
O ratio is altered, the silicate crystals exhibit significant changes in their atomic arrangements. Machatschki extended these insights to minerals in which
aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
substitutes for the
silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
atoms of the silicates. The first application of X-ray crystallography to
metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sci ...
likewise occurred in the mid-1920s. Most notably,
Linus Pauling's structure of the alloy Mg
2Sn led to his theory of the stability and structure of complex ionic crystals.
On October 17, 2012, the
Curiosity rover
''Curiosity'' is a car-sized Mars rover designed to explore the Gale crater on Mars as part of NASA's Mars Science Laboratory (MSL) mission. ''Curiosity'' was launched from Cape Canaveral (CCAFS) on November 26, 2011, at 15:02:00 UTC and ...
on the
planet Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury (planet), Mercury. In the English language, Mars is named for the Mars (mythology), Roman god of war. Mars is a terr ...
at "
Rocknest" performed the first X-ray diffraction analysis of
Martian soil. The results from the rover's
CheMin analyzer revealed the presence of several minerals, including
feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feld ...
,
pyroxenes and
olivine
The mineral olivine () is a magnesium iron silicate with the chemical formula . It is a type of nesosilicate or orthosilicate. The primary component of the Earth's upper mantle, it is a common mineral in Earth's subsurface, but weathers qui ...
, and suggested that the Martian soil in the sample was similar to the "weathered
basaltic soils" of
Hawaiian volcanoes.
Early organic and small biological molecules

The first structure of an organic compound,
hexamethylenetetramine, was solved in 1923. This was followed by several studies of long-chain
fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s, which are an important component of
biological membranes. In the 1930s, the structures of much larger molecules with two-dimensional complexity began to be solved. A significant advance was the structure of
phthalocyanine, a large planar molecule that is closely related to
porphyrin molecules important in biology, such as
heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
,
corrin and
chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to ...
.
X-ray crystallography of biological molecules took off with
Dorothy Crowfoot Hodgkin
Dorothy Mary Crowfoot Hodgkin (née Crowfoot; 12 May 1910 – 29 July 1994) was a Nobel Prize-winning British chemist who advanced the technique of X-ray crystallography to determine the structure of biomolecules, which became essential ...
, who solved the structures of
cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membr ...
(1937),
penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using ...
(1946) and
vitamin B12 (1956), for which she was awarded the
Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 1964. In 1969, she succeeded in solving the structure of
insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabol ...
, on which she worked for over thirty years.
Biological macromolecular crystallography

Crystal structures of proteins (which are irregular and hundreds of times larger than cholesterol) began to be solved in the late 1950s, beginning with the structure of
sperm whale
The sperm whale or cachalot (''Physeter macrocephalus'') is the largest of the toothed whales and the largest toothed predator. It is the only living member of the genus '' Physeter'' and one of three extant species in the sperm whale famil ...
myoglobin by
Sir John Cowdery Kendrew, for which he shared the
Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
with
Max Perutz in 1962. Since that success, over 130,000 X-ray crystal structures of proteins, nucleic acids and other biological molecules have been determined. The nearest competing method in number of structures analyzed is
nuclear magnetic resonance (NMR) spectroscopy, which has resolved less than one tenth as many. Crystallography can solve structures of arbitrarily large molecules, whereas solution-state NMR is restricted to relatively small ones (less than 70 k
Da). X-ray crystallography is used routinely to determine how a pharmaceutical drug interacts with its protein target and what changes might improve it. However, intrinsic
membrane proteins remain challenging to crystallize because they require detergents or other
denaturants to solubilize them in isolation, and such detergents often interfere with crystallization. Membrane proteins are a large component of the
genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ...
, and include many proteins of great physiological importance, such as
ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ...
s and
receptors.
Helium cryogenics are used to prevent radiation damage in protein crystals.
On the other end of the size scale, even relatively small molecules may pose challenges for the resolving power of X-ray crystallography. The structure assigned in 1991 to the antibiotic isolated from a marine organism,
diazonamide A (C
40H
34Cl
2N
6O
6, molar mass 765.65 g/mol), proved to be incorrect by the classical proof of structure: a synthetic sample was not identical to the natural product. The mistake was attributed to the inability of X-ray crystallography to distinguish between the correct -OH / -NH and the interchanged -NH
2 / -O- groups in the incorrect structure. With advances in instrumentation, however, similar groups can be distinguished using modern single-crystal X-ray diffractometers.
Despite being an invaluable tool in
structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
, protein crystallography carries some inherent problems in its methodology that hinder data interpretation. The crystal lattice, which is formed during the crystallization process, contains numerous units of the purified protein, which are densely and symmetrically packed in the crystal. When looking for a previously unknown protein, figuring out its shape and boundaries within the crystal lattice can be challenging. Proteins are usually composed of smaller subunits, and the task of distinguishing between the subunits and identifying the actual protein, can be challenging even for the experienced crystallographers. The non-biological interfaces that occur during crystallization are known as crystal-packing contacts (or simply, crystal contacts) and cannot be distinguished by crystallographic means. When a new protein structure is solved by X-ray crystallography and deposited in the
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, c ...
, its authors are requested to specify the "biological assembly" which would constitute the functional, biologically-relevant protein. However, errors, missing data and inaccurate annotations during the submission of the data, give rise to obscure structures and compromise the reliability of the database. The error rate in the case of faulty annotations alone has been reported to be upwards of 6.6% or approximately 15%, arguably a non-trivial size considering the number of deposited structures. This "interface classification problem" is typically tackled by computational approaches and has become a recognized subject in
structural bioinformatics
Structural bioinformatics is the branch of bioinformatics that is related to the analysis and prediction of the three-dimensional structure of biological macromolecules such as proteins, RNA, and DNA. It deals with generalizations about macromol ...
.
Scattering techniques
Elastic vs. inelastic scattering
X-ray crystallography is a form of
elastic scattering; the outgoing X-rays have the same energy, and thus same wavelength, as the incoming X-rays, only with altered direction. By contrast, ''inelastic scattering'' occurs when energy is transferred from the incoming X-ray to the crystal, e.g., by exciting an inner-shell electron to a higher
energy level. Such inelastic scattering reduces the energy (or increases the wavelength) of the outgoing beam. Inelastic scattering is useful for probing such excitations of matter, but not in determining the distribution of scatterers within the matter, which is the goal of X-ray crystallography.
X-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
s range in wavelength from 10 to 0.01
nanometers; a typical wavelength used for crystallography is 1
Å (0.1 nm), which is on the scale of covalent
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s and the radius of a single atom. Longer-wavelength photons (such as
ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiati ...
radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, vi ...
) would not have sufficient resolution to determine the atomic positions. At the other extreme, shorter-wavelength photons such as
gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nucleus, atomic nuclei. It consists of the shortest wavelength electromagnetic wav ...
s are difficult to produce in large numbers, difficult to focus, and interact too strongly with matter, producing
particle-antiparticle pairs. Therefore, X-rays are the "sweetspot" for wavelength when determining atomic-resolution structures from the scattering of
electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible ...
.
Other X-ray techniques
Other forms of elastic X-ray scattering besides single-crystal diffraction include
powder diffraction, Small-Angle X-ray Scattering (
SAXS
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodis ...
) and several types of X-ray
fiber diffraction, which was used by
Rosalind Franklin in determining the
double-helix structure of
DNA. In general, single-crystal X-ray diffraction offers more structural information than these other techniques; however, it requires a sufficiently large and regular crystal, which is not always available.
These scattering methods generally use ''monochromatic'' X-rays, which are restricted to a single wavelength with minor deviations. A broad spectrum of X-rays (that is, a blend of X-rays with different wavelengths) can also be used to carry out X-ray diffraction, a technique known as the Laue method. This is the method used in the original discovery of X-ray diffraction. Laue scattering provides much structural information with only a short exposure to the X-ray beam, and is therefore used in structural studies of very rapid events (
Time resolved crystallography). However, it is not as well-suited as monochromatic scattering for determining the full atomic structure of a crystal and therefore works better with crystals with relatively simple atomic arrangements.
The Laue back reflection mode records X-rays scattered backwards from a broad spectrum source. This is useful if the sample is too thick for X-rays to transmit through it. The diffracting planes in the crystal are determined by knowing that the normal to the diffracting plane bisects the angle between the incident beam and the diffracted beam. A
Greninger chart
In crystallography, a Greninger chartIt is named after Alden Buchanan Greninger (17 September 1907, Glendale, Oregon20 April 1998). is a chart that allows angular relations between zones and planes in a crystal to be directly read from an x-ray di ...
can be used to interpret the back reflection Laue photograph.
Electron and neutron diffraction
Other particles, such as electrons and
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s, may be used to produce a
diffraction pattern. Although electron, neutron, and X-ray scattering are based on different physical processes, the resulting diffraction patterns are analyzed using the same
coherent diffraction imaging
Coherent diffractive imaging (CDI) is a "lensless" technique for 2D or 3D reconstruction of the image of nanoscale structures such as nanotubes, nanocrystals, porous nanocrystalline layers, defects, potentially proteins, and more. In CDI, a highl ...
techniques.
As derived below, the electron density within the crystal and the diffraction patterns are related by a simple mathematical method, the
Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
, which allows the density to be calculated relatively easily from the patterns. However, this works only if the scattering is ''weak'', i.e., if the scattered beams are much less intense than the incoming beam. Weakly scattered beams pass through the remainder of the crystal without undergoing a second scattering event. Such re-scattered waves are called "secondary scattering" and hinder the analysis. Any sufficiently thick crystal will produce secondary scattering, but since X-rays interact relatively weakly with the electrons, this is generally not a significant concern. By contrast, electron beams may produce strong secondary scattering even for relatively thin crystals (>100 nm). Since this thickness corresponds to the diameter of many
virus
A virus is a wikt:submicroscopic, submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and ...
es, a promising direction is the electron diffraction of isolated
macromolecular assemblies, such as
viral
Viral means "relating to viruses" (small infectious agents).
Viral may also refer to:
Viral behavior, or virality
Memetic behavior likened that of a virus, for example:
* Viral marketing, the use of existing social networks to spread a marke ...
capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may ...
s and
molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for ...
s, which may be carried out with a cryo-
electron microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a ...
. Moreover, the strong interaction of electrons with matter (about 1000 times stronger than for X-rays) allows determination of the atomic structure of extremely small volumes. The field of applications for
electron crystallography ranges from bio molecules like membrane proteins over organic thin films to the complex structures of (nanocrystalline) intermetallic compounds and zeolites.
Neutron diffraction is an excellent method for structure determination, although it has been difficult to obtain intense, monochromatic beams of neutrons in sufficient quantities. Traditionally,
nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s have been used, although sources producing neutrons by
spallation are becoming increasingly available. Being uncharged, neutrons scatter much more readily from the atomic nuclei rather than from the electrons. Therefore, neutron scattering is very useful for observing the positions of light atoms with few electrons, especially
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, which is essentially invisible in the X-ray diffraction. Neutron scattering also has the remarkable property that the solvent can be made invisible by adjusting the ratio of normal
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, H
2O, and
heavy water, D
2O.
Methods
Overview of single-crystal X-ray diffraction
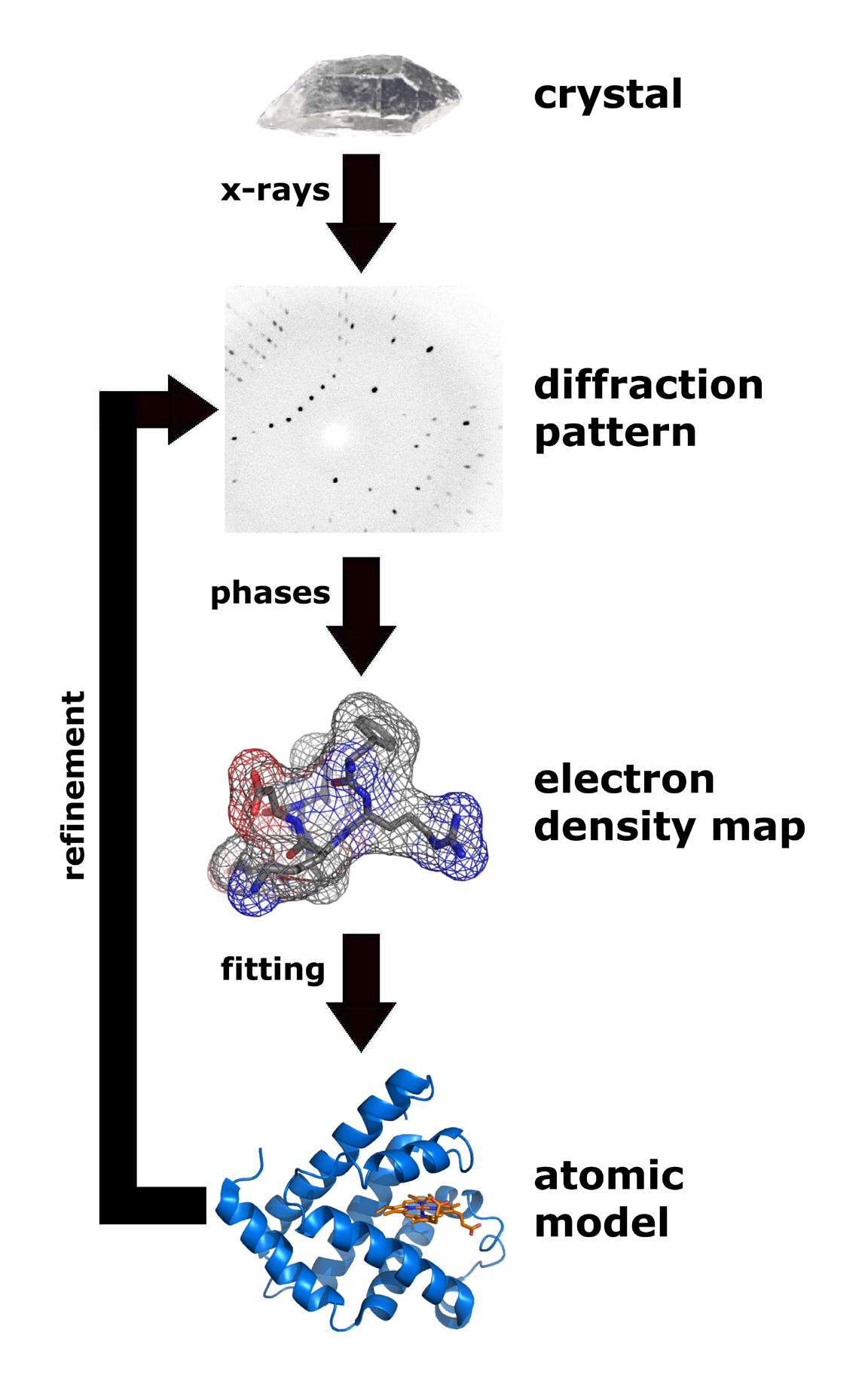
The oldest and most precise method of X-ray
crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wo ...
is ''single-crystal X-ray diffraction'', in which a beam of X-rays strikes a single crystal, producing scattered beams. When they land on a piece of film or other detector, these beams make a ''diffraction pattern'' of spots; the strengths and angles of these beams are recorded as the crystal is gradually rotated. Each spot is called a ''reflection'', since it corresponds to the reflection of the X-rays from one set of evenly spaced planes within the crystal. For single crystals of sufficient purity and regularity, X-ray diffraction data can determine the mean chemical bond lengths and angles to within a few thousandths of an angstrom and to within a few tenths of a
degree
Degree may refer to:
As a unit of measurement
* Degree (angle), a unit of angle measurement
** Degree of geographical latitude
** Degree of geographical longitude
* Degree symbol (°), a notation used in science, engineering, and mathemati ...
, respectively. The atoms in a crystal are not static, but oscillate about their mean positions, usually by less than a few tenths of an angstrom. X-ray crystallography allows measuring the size of these oscillations.
Procedure
The technique of single-crystal X-ray crystallography has three basic steps. The first—and often most difficult—step is to obtain an adequate crystal of the material under study. The crystal should be sufficiently large (typically larger than 0.1 mm in all dimensions), pure in composition and regular in structure, with no significant internal
imperfections such as cracks or
twinning.
In the second step, the crystal is placed in an intense beam of X-rays, usually of a single wavelength (''monochromatic X-rays''), producing the regular pattern of reflections. The angles and intensities of diffracted X-rays are measured, with each compound having a unique diffraction pattern. As the crystal is gradually rotated, previous reflections disappear and new ones appear; the intensity of every spot is recorded at every orientation of the crystal. Multiple data sets may have to be collected, with each set covering slightly more than half a full rotation of the crystal and typically containing tens of thousands of reflections.
In the third step, these data are combined computationally with complementary chemical information to produce and refine a model of the arrangement of atoms within the crystal. The final, refined model of the atomic arrangement—now called a ''
crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ...
''—is usually stored in a public database.
Limitations
As the crystal's repeating unit, its unit cell, becomes larger and more complex, the atomic-level picture provided by X-ray crystallography becomes less well-resolved (more "fuzzy") for a given number of observed reflections. Two limiting cases of X-ray crystallography—"small-molecule" (which includes continuous inorganic solids) and "macromolecular" crystallography—are often discerned. ''Small-molecule crystallography'' typically involves crystals with fewer than 100 atoms in their
asymmetric unit
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
; such crystal structures are usually so well resolved that the atoms can be discerned as isolated "blobs" of electron density. By contrast, ''macromolecular crystallography'' often involves tens of thousands of atoms in the unit cell. Such crystal structures are generally less well-resolved (more "smeared out"); the atoms and chemical bonds appear as tubes of electron density, rather than as isolated atoms. In general, small molecules are also easier to crystallize than macromolecules; however, X-ray crystallography has proven possible even for
virus
A virus is a wikt:submicroscopic, submicroscopic infectious agent that replicates only inside the living Cell (biology), cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and ...
es and proteins with hundreds of thousands of atoms, through improved crystallographic imaging and technology. Though normally X-ray crystallography can only be performed if the sample is in crystal form, new research has been done into sampling non-crystalline forms of samples.
Crystallization

Although crystallography can be used to characterize the disorder in an impure or irregular crystal, crystallography generally requires a pure crystal of high regularity to solve the structure of a complicated arrangement of atoms. Pure, regular crystals can sometimes be obtained from natural or synthetic materials, such as samples of
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typi ...
s, minerals or other macroscopic materials. The regularity of such crystals can sometimes be improved with macromolecular crystal
annealing and other methods. However, in many cases, obtaining a diffraction-quality crystal is the chief barrier to solving its atomic-resolution structure.
Small-molecule and macromolecular crystallography differ in the range of possible techniques used to produce diffraction-quality crystals. Small molecules generally have few degrees of conformational freedom, and may be crystallized by a wide range of methods, such as
chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
and
recrystallization. By contrast, macromolecules generally have many degrees of freedom and their crystallization must be carried out so as to maintain a stable structure. For example, proteins and larger
RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
molecules cannot be crystallized if their tertiary structure has been
unfolded; therefore, the range of crystallization conditions is restricted to solution conditions in which such molecules remain folded.

Protein crystals are almost always grown in solution. The most common approach is to lower the solubility of its component molecules very gradually; if this is done too quickly, the molecules will precipitate from solution, forming a useless dust or amorphous gel on the bottom of the container. Crystal growth in solution is characterized by two steps: ''nucleation'' of a microscopic crystallite (possibly having only 100 molecules), followed by ''growth'' of that crystallite, ideally to a diffraction-quality crystal. The solution conditions that favor the first step (nucleation) are not always the same conditions that favor the second step (subsequent growth). The crystallographer's goal is to identify solution conditions that favor the development of a single, large crystal, since larger crystals offer improved resolution of the molecule. Consequently, the solution conditions should ''disfavor'' the first step (nucleation) but ''favor'' the second (growth), so that only one large crystal forms per droplet. If nucleation is favored too much, a shower of small crystallites will form in the droplet, rather than one large crystal; if favored too little, no crystal will form whatsoever. Other approaches involves, crystallizing proteins under oil, where aqueous protein solutions are dispensed under liquid oil, and water evaporates through the layer of oil. Different oils have different evaporation permeabilities, therefore yielding changes in concentration rates from different percipient/protein mixture.
It is extremely difficult to predict good conditions for nucleation or growth of well-ordered crystals. In practice, favorable conditions are identified by ''screening''; a very large batch of the molecules is prepared, and a wide variety of crystallization solutions are tested. Hundreds, even thousands, of solution conditions are generally tried before finding the successful one. The various conditions can use one or more physical mechanisms to lower the solubility of the molecule; for example, some may change the pH, some contain salts of the
Hofmeister series
The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who stud ...
or chemicals that lower the dielectric constant of the solution, and still others contain large polymers such as
polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular w ...
that drive the molecule out of solution by entropic effects. It is also common to try several temperatures for encouraging crystallization, or to gradually lower the temperature so that the solution becomes supersaturated. These methods require large amounts of the target molecule, as they use high concentration of the molecule(s) to be crystallized. Due to the difficulty in obtaining such large quantities (
milligrams) of crystallization-grade protein, robots have been developed that are capable of accurately dispensing crystallization trial drops that are in the order of 100
nanoliters in volume. This means that 10-fold less protein is used per experiment when compared to crystallization trials set up by hand (in the order of 1
microliter).
Several factors are known to inhibit or mar crystallization. The growing crystals are generally held at a constant temperature and protected from shocks or vibrations that might disturb their crystallization. Impurities in the molecules or in the crystallization solutions are often inimical to crystallization. Conformational flexibility in the molecule also tends to make crystallization less likely, due to entropy. Molecules that tend to self-assemble into regular helices are often unwilling to assemble into crystals. Crystals can be marred by
twinning, which can occur when a unit cell can pack equally favorably in multiple orientations; although recent advances in computational methods may allow solving the structure of some twinned crystals. Having failed to crystallize a target molecule, a crystallographer may try again with a slightly modified version of the molecule; even small changes in molecular properties can lead to large differences in crystallization behavior.
Data collection
Mounting the crystal
The crystal is mounted for measurements so that it may be held in the X-ray beam and rotated. There are several methods of mounting. In the past, crystals were loaded into glass capillaries with the crystallization solution (the
mother liquor). Nowadays, crystals of small molecules are typically attached with oil or glue to a glass fiber or a loop, which is made of nylon or plastic and attached to a solid rod. Protein crystals are scooped up by a loop, then flash-frozen with
liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wi ...
. This freezing reduces the radiation damage of the X-rays, as well as the noise in the Bragg peaks due to thermal motion (the Debye-Waller effect). However, untreated protein crystals often crack if flash-frozen; therefore, they are generally pre-soaked in a cryoprotectant solution before freezing. Unfortunately, this pre-soak may itself cause the crystal to crack, ruining it for crystallography. Generally, successful cryo-conditions are identified by trial and error.
The capillary or loop is mounted on a
goniometer, which allows it to be positioned accurately within the X-ray beam and rotated. Since both the crystal and the beam are often very small, the crystal must be centered within the beam to within ~25 micrometers accuracy, which is aided by a camera focused on the crystal. The most common type of goniometer is the "kappa goniometer", which offers three angles of rotation: the ω angle, which rotates about an axis perpendicular to the beam; the κ angle, about an axis at ~50° to the ω axis; and, finally, the φ angle about the loop/capillary axis. When the κ angle is zero, the ω and φ axes are aligned. The κ rotation allows for convenient mounting of the crystal, since the arm in which the crystal is mounted may be swung out towards the crystallographer. The oscillations carried out during data collection (mentioned below) involve the ω axis only. An older type of goniometer is the four-circle goniometer, and its relatives such as the six-circle goniometer.
X-ray sources
=Rotating anode
=
Small scale crystallography can be done with a local
X-ray tube
An X-ray tube is a vacuum tube that converts electrical input power into X-rays. The availability of this controllable source of X-rays created the field of radiography, the imaging of partly opaque objects with penetrating radiation. In contrast ...
source, typically coupled with an
image plate detector. These have the advantage of being relatively inexpensive and easy to maintain, and allow for quick screening and collection of samples. However, the wavelength of the light produced is limited by the availability of different
anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemoni ...
materials. Furthermore, the intensity is limited by the power applied and cooling capacity available to avoid melting the anode. In such systems, electrons are boiled off of a cathode and accelerated through a strong electric potential of ~50
kV; having reached a high speed, the electrons collide with a metal plate, emitting ''
bremsstrahlung'' and some strong spectral lines corresponding to the excitation of
inner-shell electrons of the metal. The most common metal used is
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
, which can be kept cool easily, due to its high
thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
, and which produces strong
Kα and K
β lines. The K
β line is sometimes suppressed with a thin (~10 µm) nickel foil. The simplest and cheapest variety of sealed X-ray tube has a stationary anode (the
Crookes tube
A Crookes tube (also Crookes–Hittorf tube) is an early experimental electrical discharge tube, with partial vacuum, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were ...
) and run with ~2
kW of electron beam power. The more expensive variety has a
rotating-anode type source that run with ~14 kW of e-beam power.
X-rays are generally filtered (by use of
X-ray filters) to a single wavelength (made monochromatic) and
collimated to a single direction before they are allowed to strike the crystal. The filtering not only simplifies the data analysis, but also removes radiation that degrades the crystal without contributing useful information. Collimation is done either with a collimator (basically, a long tube) or with a clever arrangement of gently curved mirrors. Mirror systems are preferred for small crystals (under 0.3 mm) or with large unit cells (over 150 Å).
Rotating anodes were used by
Joanna (Joka) Maria Vandenberg in the first experiments that demonstrated the power of X rays for quick (in real time production) screening of large
InGaAsP Indium gallium arsenide phosphide () is a quaternary compound semiconductor material, an alloy of gallium arsenide, gallium phosphide, indium arsenide, or indium phosphide. This compound has applications in photonic devices, due to the ability to ta ...
thin film
A thin film is a layer of material ranging from fractions of a nanometer ( monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many a ...
wafers for
quality control of
quantum well lasers.
=Microfocus tube
=
A more recent development is the
microfocus tube, which can deliver at least as high a beam flux (after collimation) as rotating-anode sources but only require a beam power of a few tens or hundreds of watts rather than requiring several kilowatts.
=Synchrotron radiation
=
Synchrotron radiation sources are some of the brightest light sources on earth and are some of the most powerful tools available to X-ray crystallographers. X-ray beams generated in large machines called
synchrotrons which accelerate electrically charged particles, often electrons, to nearly the speed of light and confine them in a (roughly) circular loop using magnetic fields.
Synchrotrons are generally national facilities, each with several dedicated
beamlines where data is collected without interruption. Synchrotrons were originally designed for use by high-energy physicists studying
subatomic particles and
cosmic
Cosmic commonly refers to:
* The cosmos, a concept of the universe
Cosmic may also refer to:
Media
* ''Cosmic'' (album), an album by Bazzi
* Afro/Cosmic music
* "Cosmic", a song by Kylie Minogue from the album '' X''
* CosM.i.C, a member of ...
phenomena. The largest component of each synchrotron is its electron storage ring. This ring is actually not a perfect circle, but a many-sided polygon. At each corner of the polygon, or sector, precisely aligned magnets bend the electron stream. As the electrons' path is bent, they emit bursts of energy in the form of X-rays.
Using synchrotron radiation frequently has specific requirements for X-ray crystallography. The intense
ionizing radiation can cause
radiation damage to samples, particularly macromolecular crystals.
Cryo crystallography protects the sample from radiation damage, by freezing the crystal at
liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wi ...
temperatures (~100
K). Cryocrystallography methods are applied to home source rotating anode sources as well. However, synchrotron radiation frequently has the advantage of user-selectable wavelengths, allowing for
anomalous scattering experiments which maximizes anomalous signal. This is critical in experiments such as
single wavelength anomalous dispersion (SAD) and
multi-wavelength anomalous dispersion (MAD).
=Free-electron laser
=
Free-electron laser
A free-electron laser (FEL) is a (fourth generation) light source producing extremely brilliant and short pulses of radiation. An FEL functions and behaves in many ways like a laser, but instead of using stimulated emission from atomic or molecul ...
s have been developed for use in X-ray crystallography. These are the brightest X-ray sources currently available; with the X-rays coming in
femtosecond bursts. The intensity of the source is such that atomic resolution diffraction patterns can be resolved for crystals otherwise too small for collection. However, the intense light source also destroys the sample, requiring multiple crystals to be shot. As each crystal is randomly oriented in the beam, hundreds of thousands of individual diffraction images must be collected in order to get a complete data set. This method,
serial femtosecond crystallography Serial femtosecond crystallography (SFX) is a form of X-ray crystallography developed for use at X-ray free-electron lasers (XFELs). Single pulses at free-electron lasers are bright enough to generate resolvable Bragg diffraction from sub-micron cr ...
, has been used in solving the structure of a number of protein crystal structures, sometimes noting differences with equivalent structures collected from synchrotron sources.
Recording the reflections

When a crystal is mounted and exposed to an intense beam of X-rays, it scatters the X-rays into a pattern of spots or ''reflections'' that can be observed on a screen behind the crystal. A similar pattern may be seen by shining a
laser pointer at a
compact disc
The compact disc (CD) is a digital optical disc data storage format that was co-developed by Philips and Sony to store and play digital audio recordings. In August 1982, the first compact disc was manufactured. It was then released in Octo ...
. The relative intensities of these spots provide the information to determine the arrangement of molecules within the crystal in atomic detail. The intensities of these reflections may be recorded with
photographic film, an area detector (such as a
pixel detector) or with a
charge-coupled device
A charge-coupled device (CCD) is an integrated circuit containing an array of linked, or coupled, capacitors. Under the control of an external circuit, each capacitor can transfer its electric charge to a neighboring capacitor. CCD sensors are ...
(CCD) image sensor. The peaks at small angles correspond to low-resolution data, whereas those at high angles represent high-resolution data; thus, an upper limit on the eventual resolution of the structure can be determined from the first few images. Some measures of diffraction quality can be determined at this point, such as the
mosaicity
In crystallography, mosaicity is a measure of the spread of crystal plane orientations. A mosaic crystal is an idealized model of an imperfect crystal, imagined to consist of numerous small perfect crystals (crystallites) that are to some extent ra ...
of the crystal and its overall disorder, as observed in the peak widths. Some pathologies of the crystal that would render it unfit for solving the structure can also be diagnosed quickly at this point.
One image of spots is insufficient to reconstruct the whole crystal; it represents only a small slice of the full Fourier transform. To collect all the necessary information, the crystal must be rotated step-by-step through 180°, with an image recorded at every step; actually, slightly more than 180° is required to cover
reciprocal space, due to the curvature of the
Ewald sphere. However, if the crystal has a higher symmetry, a smaller angular range such as 90° or 45° may be recorded. The rotation axis should be changed at least once, to avoid developing a "blind spot" in reciprocal space close to the rotation axis. It is customary to rock the crystal slightly (by 0.5–2°) to catch a broader region of reciprocal space.
Multiple data sets may be necessary for certain
phasing methods. For example,
multi-wavelength anomalous dispersion phasing requires that the scattering be recorded at least three (and usually four, for redundancy) wavelengths of the incoming X-ray radiation. A single crystal may degrade too much during the collection of one data set, owing to radiation damage; in such cases, data sets on multiple crystals must be taken.
Data analysis
Crystal symmetry, unit cell, and image scaling
The recorded series of two-dimensional diffraction patterns, each corresponding to a different crystal orientation, is converted into a three-dimensional model of the electron density; the conversion uses the mathematical technique of Fourier transforms, which is explained
below
Below may refer to:
*Earth
* Ground (disambiguation)
* Soil
* Floor
* Bottom (disambiguation)
* Less than
*Temperatures below freezing
* Hell or underworld
People with the surname
* Ernst von Below (1863–1955), German World War I general
* Fr ...
. Each spot corresponds to a different type of variation in the electron density; the crystallographer must determine ''which'' variation corresponds to ''which'' spot (''indexing''), the relative strengths of the spots in different images (''merging and scaling'') and how the variations should be combined to yield the total electron density (''phasing'').
Data processing begins with ''indexing'' the reflections. This means identifying the dimensions of the unit cell and which image peak corresponds to which position in reciprocal space. A byproduct of indexing is to determine the symmetry of the crystal, i.e., its ''
space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it ...
''. Some space groups can be eliminated from the beginning. For example, reflection symmetries cannot be observed in chiral molecules; thus, only 65 space groups of 230 possible are allowed for protein molecules which are almost always chiral. Indexing is generally accomplished using an ''autoindexing'' routine. Having assigned symmetry, the data is then ''integrated''. This converts the hundreds of images containing the thousands of reflections into a single file, consisting of (at the very least) records of the
Miller index
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and '' ...
of each reflection, and an intensity for each reflection (at this state the file often also includes error estimates and measures of partiality (what part of a given reflection was recorded on that image)).
A full data set may consist of hundreds of separate images taken at different orientations of the crystal. The first step is to merge and scale these various images, that is, to identify which peaks appear in two or more images (''merging'') and to scale the relative images so that they have a consistent intensity scale. Optimizing the intensity scale is critical because the relative intensity of the peaks is the key information from which the structure is determined. The repetitive technique of crystallographic data collection and the often high symmetry of crystalline materials cause the diffractometer to record many symmetry-equivalent reflections multiple times. This allows calculating the symmetry-related
R-factor, a reliability index based upon how similar are the measured intensities of symmetry-equivalent reflections, thus assessing the quality of the data.
Initial phasing
The data collected from a diffraction experiment is a
reciprocal space representation of the crystal lattice. The position of each diffraction 'spot' is governed by the size and shape of the unit cell, and the inherent
symmetry within the crystal. The intensity of each diffraction 'spot' is recorded, and this intensity is proportional to the square of the ''structure factor''
amplitude
The amplitude of a periodic variable is a measure of its change in a single period (such as time or spatial period). The amplitude of a non-periodic signal is its magnitude compared with a reference value. There are various definitions of a ...
. The
structure factor is a
complex number
In mathematics, a complex number is an element of a number system that extends the real numbers with a specific element denoted , called the imaginary unit and satisfying the equation i^= -1; every complex number can be expressed in the for ...
containing information relating to both the
amplitude
The amplitude of a periodic variable is a measure of its change in a single period (such as time or spatial period). The amplitude of a non-periodic signal is its magnitude compared with a reference value. There are various definitions of a ...
and
phase of a
wave
In physics, mathematics, and related fields, a wave is a propagating dynamic disturbance (change from equilibrium) of one or more quantities. Waves can be periodic, in which case those quantities oscillate repeatedly about an equilibrium (r ...
. In order to obtain an interpretable ''electron density map'', both amplitude and phase must be known (an electron density map allows a crystallographer to build a starting model of the molecule). The phase cannot be directly recorded during a diffraction experiment: this is known as the
phase problem. Initial phase estimates can be obtained in a variety of ways:
* ''
Ab initio'' phasing or
direct methods – This is usually the method of choice for small molecules (<1000 non-hydrogen atoms), and has been used successfully to solve the phase problems for small proteins. If the resolution of the data is better than 1.4 Å (140
pm),
direct methods can be used to obtain phase information, by exploiting known phase relationships between certain groups of reflections.
*
Molecular replacement – if a related structure is known, it can be used as a search model in molecular replacement to determine the orientation and position of the molecules within the unit cell. The phases obtained this way can be used to generate electron density maps.
*
Anomalous X-ray scattering (''
MAD or
SAD phasing'') – the X-ray wavelength may be scanned past an absorption edge of an atom, which changes the scattering in a known way. By recording full sets of reflections at three different wavelengths (far below, far above and in the middle of the absorption edge) one can solve for the substructure of the anomalously diffracting atoms and hence the structure of the whole molecule. The most popular method of incorporating anomalous scattering atoms into proteins is to express the protein in a
methionine auxotroph (a host incapable of synthesizing methionine) in a media rich in seleno-methionine, which contains
selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and telluriu ...
atoms. A multi-wavelength anomalous dispersion (MAD) experiment can then be conducted around the absorption edge, which should then yield the position of any methionine residues within the protein, providing initial phases.
* Heavy atom methods (
multiple isomorphous replacement) – If electron-dense metal atoms can be introduced into the crystal,
direct methods or
Patterson-space methods can be used to determine their location and to obtain initial phases. Such heavy atoms can be introduced either by soaking the crystal in a heavy atom-containing solution, or by co-crystallization (growing the crystals in the presence of a heavy atom). As in multi-wavelength anomalous dispersion phasing, the changes in the scattering amplitudes can be interpreted to yield the phases. Although this is the original method by which protein crystal structures were solved, it has largely been superseded by multi-wavelength anomalous dispersion phasing with selenomethionine.
[
]
Model building and phase refinement
 Having obtained initial phases, an initial model can be built. The atomic positions in the model and their respective Debye-Waller factors (or B-factors, accounting for the thermal motion of the atom) can be refined to fit the observed diffraction data, ideally yielding a better set of phases. A new model can then be fit to the new electron density map and successive rounds of refinement is carried out. This iterative process continues until the correlation between the diffraction data and the model is maximized. The agreement is measured by an ''R''-factor defined as
:
where ''F'' is the structure factor. A similar quality criterion is ''R''free, which is calculated from a subset (~10%) of reflections that were not included in the structure refinement. Both ''R'' factors depend on the resolution of the data. As a rule of thumb, ''R''free should be approximately the resolution in angstroms divided by 10; thus, a data-set with 2 Å resolution should yield a final ''R''free ~ 0.2. Chemical bonding features such as stereochemistry, hydrogen bonding and distribution of bond lengths and angles are complementary measures of the model quality. Phase bias is a serious problem in such iterative model building. ''Omit maps'' are a common technique used to check for this.
It may not be possible to observe every atom in the asymmetric unit. In many cases, Crystallographic disorder smears the electron density map. Weakly scattering atoms such as hydrogen are routinely invisible. It is also possible for a single atom to appear multiple times in an electron density map, e.g., if a protein sidechain has multiple (<4) allowed conformations. In still other cases, the crystallographer may detect that the covalent structure deduced for the molecule was incorrect, or changed. For example, proteins may be cleaved or undergo post-translational modifications that were not detected prior to the crystallization.
Having obtained initial phases, an initial model can be built. The atomic positions in the model and their respective Debye-Waller factors (or B-factors, accounting for the thermal motion of the atom) can be refined to fit the observed diffraction data, ideally yielding a better set of phases. A new model can then be fit to the new electron density map and successive rounds of refinement is carried out. This iterative process continues until the correlation between the diffraction data and the model is maximized. The agreement is measured by an ''R''-factor defined as
:
where ''F'' is the structure factor. A similar quality criterion is ''R''free, which is calculated from a subset (~10%) of reflections that were not included in the structure refinement. Both ''R'' factors depend on the resolution of the data. As a rule of thumb, ''R''free should be approximately the resolution in angstroms divided by 10; thus, a data-set with 2 Å resolution should yield a final ''R''free ~ 0.2. Chemical bonding features such as stereochemistry, hydrogen bonding and distribution of bond lengths and angles are complementary measures of the model quality. Phase bias is a serious problem in such iterative model building. ''Omit maps'' are a common technique used to check for this.
It may not be possible to observe every atom in the asymmetric unit. In many cases, Crystallographic disorder smears the electron density map. Weakly scattering atoms such as hydrogen are routinely invisible. It is also possible for a single atom to appear multiple times in an electron density map, e.g., if a protein sidechain has multiple (<4) allowed conformations. In still other cases, the crystallographer may detect that the covalent structure deduced for the molecule was incorrect, or changed. For example, proteins may be cleaved or undergo post-translational modifications that were not detected prior to the crystallization.
Disorder
A common challenge in refinement of crystal structures results from crystallographic disorder. Disorder can take many forms but in general involves the coexistence of two or more species or conformations. Failure to recognize disorder results in flawed interpretation. Pitfalls from improper modeling of disorder are illustrated by the discounted hypothesis of bond stretch isomerism. Disorder is modelled with respect to the relative population of the components, often only two, and their identity. In structures of large molecules and ions, solvent and counterions are often disordered.
Applied computational data analysis
The use of computational methods for the powder X-ray diffraction data analysis is now generalized. It typically compares the experimental data to the simulated diffractogram of a model structure, taking into account the instrumental parameters, and refines the structural or microstructural parameters of the model using least squares
The method of least squares is a standard approach in regression analysis to approximate the solution of overdetermined systems (sets of equations in which there are more equations than unknowns) by minimizing the sum of the squares of the r ...
based minimization algorithm. Most available tools allowing phase identification and structural refinement are based on the Rietveld method, some of them being open and free software such as FullProf Suite, Jana2006, MAUD, Rietan, GSAS, etc. while others are available under commercials licenses such as Diffrac.Suite TOPAS, Match!, etc. Most of these tools also allow Le Bail refinement (also referred to as profile matching), that is, refinement of the cell parameters based on the Bragg peaks positions and peak profiles, without taking into account the crystallographic structure by itself. More recent tools allow the refinement of both structural and microstructural data, such as the FAULTS program included in the FullProf Suite, which allows the refinement of structures with planar defects (e.g. stacking faults, twinnings, intergrowths).
Deposition of the structure
Once the model of a molecule's structure has been finalized, it is often deposited in a crystallographic database such as the Cambridge Structural Database (for small molecules), the Inorganic Crystal Structure Database (ICSD) (for inorganic compounds) or the Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, c ...
(for protein and sometimes nucleic acids). Many structures obtained in private commercial ventures to crystallize medicinally relevant proteins are not deposited in public crystallographic databases.
Diffraction theory
The main goal of X-ray crystallography is to determine the density of electrons ''f''(r) throughout the crystal, where r represents the three-dimensional position vector within the crystal. To do this, X-ray scattering is used to collect data about its Fourier transform ''F''(q), which is inverted mathematically to obtain the density defined in real space, using the formula
:
where the integral
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along with ...
is taken over all values of q. The three-dimensional real vector q represents a point in reciprocal space, that is, to a particular oscillation in the electron density as one moves in the direction in which q points. The length of q corresponds to divided by the wavelength of the oscillation. The corresponding formula for a Fourier transform will be used below
:
where the integral
In mathematics, an integral assigns numbers to functions in a way that describes displacement, area, volume, and other concepts that arise by combining infinitesimal data. The process of finding integrals is called integration. Along with ...
is summed over all possible values of the position vector r within the crystal.
The Fourier transform ''F''(q) is generally a complex number
In mathematics, a complex number is an element of a number system that extends the real numbers with a specific element denoted , called the imaginary unit and satisfying the equation i^= -1; every complex number can be expressed in the for ...
, and therefore has a magnitude , ''F''(q), and a phase ''φ''(q) related by the equation
:
The intensities of the reflections observed in X-ray diffraction give us the magnitudes , ''F''(q), but not the phases ''φ''(q). To obtain the phases, full sets of reflections are collected with known alterations to the scattering, either by modulating the wavelength past a certain absorption edge or by adding strongly scattering (i.e., electron-dense) metal atoms such as mercury. Combining the magnitudes and phases yields the full Fourier transform ''F''(q), which may be inverted to obtain the electron density ''f''(r).
Crystals are often idealized as being ''perfectly'' periodic. In that ideal case, the atoms are positioned on a perfect lattice, the electron density is perfectly periodic, and the Fourier transform ''F''(q) is zero except when q belongs to the reciprocal lattice (the so-called ''Bragg peaks''). In reality, however, crystals are not perfectly periodic; atoms vibrate about their mean position, and there may be disorder of various types, such as mosaicity
In crystallography, mosaicity is a measure of the spread of crystal plane orientations. A mosaic crystal is an idealized model of an imperfect crystal, imagined to consist of numerous small perfect crystals (crystallites) that are to some extent ra ...
, dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to ...
s, various point defects, and heterogeneity in the conformation of crystallized molecules. Therefore, the Bragg peaks have a finite width and there may be significant ''diffuse scattering'', a continuum of scattered X-rays that fall between the Bragg peaks.
Intuitive understanding by Bragg's law
An intuitive understanding of X-ray diffraction can be obtained from the Bragg model of diffraction. In this model, a given reflection is associated with a set of evenly spaced sheets running through the crystal, usually passing through the centers of the atoms of the crystal lattice. The orientation of a particular set of sheets is identified by its three Miller indices (''h'', ''k'', ''l''), and let their spacing be noted by ''d''. William Lawrence Bragg proposed a model in which the incoming X-rays are scattered specularly (mirror-like) from each plane; from that assumption, X-rays scattered from adjacent planes will combine constructively ( constructive interference) when the angle θ between the plane and the X-ray results in a path-length difference that is an integer multiple ''n'' of the X-ray wavelength λ.
:
A reflection is said to be ''indexed'' when its Miller indices (or, more correctly, its reciprocal lattice vector components) have been identified from the known wavelength and the scattering angle 2θ. Such indexing gives the unit-cell parameters, the lengths and angles of the unit-cell, as well as its space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it ...
. Since Bragg's law does not interpret the relative intensities of the reflections, however, it is generally inadequate to solve for the arrangement of atoms within the unit-cell; for that, a Fourier transform method must be carried out.
Scattering as a Fourier transform
The incoming X-ray beam has a polarization and should be represented as a vector wave; however, for simplicity, let it be represented here as a scalar wave. We also ignore the complication of the time dependence of the wave and just concentrate on the wave's spatial dependence. Plane waves can be represented by a wave vector kin, and so the strength of the incoming wave at time ''t'' = 0 is given by
:
At position r within the sample, let there be a density of scatterers ''f''(r); these scatterers should produce a scattered spherical wave of amplitude proportional to the local amplitude of the incoming wave times the number of scatterers in a small volume ''dV'' about r
:
where ''S'' is the proportionality constant.
Consider the fraction of scattered waves that leave with an outgoing wave-vector of kout and strike the screen at rscreen. Since no energy is lost (elastic, not inelastic scattering), the wavelengths are the same as are the magnitudes of the wave-vectors , kin, =, kout, . From the time that the photon is scattered at r until it is absorbed at rscreen, the photon undergoes a change in phase
:
The net radiation arriving at rscreen is the sum of all the scattered waves throughout the crystal
:
which may be written as a Fourier transform
:
where q = kout – kin. The measured intensity of the reflection will be square of this amplitude
:
Friedel and Bijvoet mates
For every reflection corresponding to a point q in the reciprocal space, there is another reflection of the same intensity at the opposite point -q. This opposite reflection is known as the ''Friedel mate'' of the original reflection. This symmetry results from the mathematical fact that the density of electrons ''f''(r) at a position r is always a real number
In mathematics, a real number is a number that can be used to measurement, measure a ''continuous'' one-dimensional quantity such as a distance, time, duration or temperature. Here, ''continuous'' means that values can have arbitrarily small var ...
. As noted above, ''f''(r) is the inverse transform of its Fourier transform ''F''(q); however, such an inverse transform is a complex number
In mathematics, a complex number is an element of a number system that extends the real numbers with a specific element denoted , called the imaginary unit and satisfying the equation i^= -1; every complex number can be expressed in the for ...
in general. To ensure that ''f''(r) is real, the Fourier transform ''F''(q) must be such that the Friedel mates ''F''(−q) and ''F''(q) are complex conjugate
In mathematics, the complex conjugate of a complex number is the number with an equal real part and an imaginary part equal in magnitude but opposite in sign. That is, (if a and b are real, then) the complex conjugate of a + bi is equal to a - ...
s of one another. Thus, ''F''(−q) has the same magnitude as ''F''(q) but they have the opposite phase, i.e., ''φ''(q) = −''φ''(q)
:
The equality of their magnitudes ensures that the Friedel mates have the same intensity , ''F'', 2. This symmetry allows one to measure the full Fourier transform from only half the reciprocal space, e.g., by rotating the crystal slightly more than 180° instead of a full 360° revolution. In crystals with significant symmetry, even more reflections may have the same intensity (Bijvoet mates); in such cases, even less of the reciprocal space may need to be measured. In favorable cases of high symmetry, sometimes only 90° or even only 45° of data are required to completely explore the reciprocal space.
The Friedel-mate constraint can be derived from the definition of the inverse Fourier transform
:
Since Euler's formula states that ei''x'' = cos(''x'') + i sin(''x''), the inverse Fourier transform can be separated into a sum of a purely real part and a purely imaginary part
:
The function ''f''(r) is real if and only if the second integral ''I''sin is zero for all values of r. In turn, this is true if and only if the above constraint is satisfied
:
since ''I''sin = −''I''sin implies that ''I''sin = 0.
Ewald's sphere
 Each X-ray diffraction image represents only a slice, a spherical slice of reciprocal space, as may be seen by the Ewald sphere construction. Both kout and kin have the same length, due to the elastic scattering, since the wavelength has not changed. Therefore, they may be represented as two radial vectors in a sphere in reciprocal space, which shows the values of q that are sampled in a given diffraction image. Since there is a slight spread in the incoming wavelengths of the incoming X-ray beam, the values of, ''F''(q), can be measured only for q vectors located between the two spheres corresponding to those radii. Therefore, to obtain a full set of Fourier transform data, it is necessary to rotate the crystal through slightly more than 180°, or sometimes less if sufficient symmetry is present. A full 360° rotation is not needed because of a symmetry intrinsic to the Fourier transforms of real functions (such as the electron density), but "slightly more" than 180° is needed to cover all of reciprocal space within a given resolution because of the curvature of the Ewald sphere. In practice, the crystal is rocked by a small amount (0.25–1°) to incorporate reflections near the boundaries of the spherical Ewald's shells.
Each X-ray diffraction image represents only a slice, a spherical slice of reciprocal space, as may be seen by the Ewald sphere construction. Both kout and kin have the same length, due to the elastic scattering, since the wavelength has not changed. Therefore, they may be represented as two radial vectors in a sphere in reciprocal space, which shows the values of q that are sampled in a given diffraction image. Since there is a slight spread in the incoming wavelengths of the incoming X-ray beam, the values of, ''F''(q), can be measured only for q vectors located between the two spheres corresponding to those radii. Therefore, to obtain a full set of Fourier transform data, it is necessary to rotate the crystal through slightly more than 180°, or sometimes less if sufficient symmetry is present. A full 360° rotation is not needed because of a symmetry intrinsic to the Fourier transforms of real functions (such as the electron density), but "slightly more" than 180° is needed to cover all of reciprocal space within a given resolution because of the curvature of the Ewald sphere. In practice, the crystal is rocked by a small amount (0.25–1°) to incorporate reflections near the boundaries of the spherical Ewald's shells.
Patterson function
A well-known result of Fourier transforms is the autocorrelation
Autocorrelation, sometimes known as serial correlation in the discrete time case, is the correlation of a signal with a delayed copy of itself as a function of delay. Informally, it is the similarity between observations of a random variable ...
theorem, which states that the autocorrelation ''c''(r) of a function ''f''(r)
:
has a Fourier transform ''C''(q) that is the squared magnitude of ''F''(q)
:
Therefore, the autocorrelation function ''c''(r) of the electron density (also known as the ''Patterson function'') can be computed directly from the reflection intensities, without computing the phases. In principle, this could be used to determine the crystal structure directly; however, it is difficult to realize in practice. The autocorrelation function corresponds to the distribution of vectors between atoms in the crystal; thus, a crystal of ''N'' atoms in its unit cell may have ''N''(''N'' − 1) peaks in its Patterson function. Given the inevitable errors in measuring the intensities, and the mathematical difficulties of reconstructing atomic positions from the interatomic vectors, this technique is rarely used to solve structures, except for the simplest crystals.
Advantages of a crystal
In principle, an atomic structure could be determined from applying X-ray scattering to non-crystalline samples, even to a single molecule. However, crystals offer a much stronger signal due to their periodicity. A crystalline sample is by definition periodic; a crystal is composed of many unit cells repeated indefinitely in three independent directions. Such periodic systems have a Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
that is concentrated at periodically repeating points in reciprocal space known as ''Bragg peaks''; the Bragg peaks correspond to the reflection spots observed in the diffraction image. Since the amplitude at these reflections grows linearly with the number ''N'' of scatterers, the observed ''intensity'' of these spots should grow quadratically, like ''N''2. In other words, using a crystal concentrates the weak scattering of the individual unit cells into a much more powerful, coherent reflection that can be observed above the noise. This is an example of constructive interference.
In a liquid, powder or amorphous sample, molecules within that sample are in random orientations. Such samples have a continuous Fourier spectrum that uniformly spreads its amplitude thereby reducing the measured signal intensity, as is observed in SAXS
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodis ...
. More importantly, the orientational information is lost. Although theoretically possible, it is experimentally difficult to obtain atomic-resolution structures of complicated, asymmetric molecules from such rotationally averaged data. An intermediate case is fiber diffraction in which the subunits are arranged periodically in at least one dimension.
Nobel Prizes involving X-ray crystallography
Applications
X-ray diffraction has wide and various applications in the chemical, biochemical, physical, material and mineralogical sciences. Laue claimed in 1937 that the technique "has extended the power of observing minute structure ten thousand times beyond that given us by the microscope".
Drug identification
X-ray diffraction has been used for the identification of antibiotic drugs such as: eight β-lactam ( ampicillin sodium, penicillin G procaine, cefalexin
Cefalexin, also spelled cephalexin, is an antibiotic that can treat a number of bacterial infections. It kills gram-positive and some gram-negative bacteria by disrupting the growth of the bacterial cell wall. Cefalexin is a beta-lactam ant ...
, ampicillin trihydrate, benzathine penicillin, benzylpenicillin sodium
Benzylpenicillin, also known as penicillin G (PenG) or BENPEN, and in military slang "Peanut Butter Shot" is an antibiotic used to treat a number of bacterial infections. This includes pneumonia, strep throat, syphilis, necrotizing enterocolitis ...
, cefotaxime sodium
Cefotaxime is an antibiotic used to treat a number of bacterial infections in human, other animals and plant tissue culture. Specifically in humans it is used to treat joint infections, pelvic inflammatory disease, meningitis, pneumonia, urinary ...
, Ceftriaxone sodium
Ceftriaxone, sold under the brand name Rocephin, is a third-generation cephalosporin antibiotic used for the treatment of a number of bacterial infections. These include middle ear infections, endocarditis, meningitis, pneumonia, bone and joint ...
), three tetracycline ( doxycycline hydrochloride, oxytetracycline dehydrate, tetracycline hydrochloride) and two macrolide
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. M ...
(azithromycin
Azithromycin, sold under the brand names Zithromax (in oral form) and Azasite (as an eye drop), is an antibiotic medication used for the treatment of a number of bacterial infections. This includes middle ear infections, strep throat, pneum ...
, erythromycin estolate
Erythromycin is an antibiotic used for the treatment of a number of bacterial infections. This includes respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis. It may also be used duri ...
) antibiotic drugs. Each of these drugs has a unique X-Ray Diffraction (XRD) pattern that makes their identification possible.
Characterization of nanomaterials, textile fibers and polymers
Forensic examination of any trace evidence is based upon Locard's exchange principle
In forensic science, Locard's principle holds that the perpetrator of a crime will bring something into the crime scene and leave with something from it, and that both can be used as forensic evidence. Dr. Edmond Locard (1877–1966) was a pion ...
. This states that "every contact leaves a trace". In practice, even though a transfer of material has taken place, it may be impossible to detect, because the amount transferred is very small.
XRD has proven its role in the advancement of nanomaterial research. It is one of the primary characterization tools and provides information about the structural properties of various nanomaterials in both powder and thin-film form.
Textile fibers are a mixture of crystalline and amorphous substances. Therefore, the measurement of the degree of crystalline gives useful data in the characterization of fibers using X-ray diffractometry. It has been reported that X-ray diffraction was used to identify of a "crystalline" deposit which was found on a chair. The deposit was found to be amorphous, but the diffraction pattern present matched that of polymethylmethacrylate. Pyrolysis mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
later identified the deposit as polymethylcyanoacrylaon of Boin crystal parameters.
Investigation of bones
Heating or burning of bones causes recognizable changes in the bone mineral that can be detected using XRD techniques. During the first 15 minutes of heating at 500 °C or above, the bone crystals began to change. At higher temperatures, thickness and shape of crystals of bones appear stabilized, but when the samples were heated at a lower temperature or for a shorter period, XRD traces showed extreme changes in crystal parameters.
Integrated circuits
X-ray diffraction has been demonstrated as a method for investigating the complex structure of integrated circuits
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Transistor count, Large ...
.
See also
* Beevers–Lipson strip
* Bragg diffraction
* Crystallographic database
* Crystallographic point groups
* Difference density map
* Electron diffraction
* Energy Dispersive X-Ray Diffraction
Energy-dispersive X-ray diffraction (EDXRD) is an analytical technique for characterizing materials. It differs from conventional X-ray diffraction by using polychromatic photons as the source and is usually operated at a fixed angle. With no nee ...
* Flack parameter In X-ray crystallography, the Flack parameter is a factor used to estimate the absolute configuration of a structural model determined by single-crystal structure analysis.
In this approach, one determines the absolute structure of a noncentrosymm ...
* Henderson limit
* International Year of Crystallography
* John Desmond Bernal
* Multipole density formalism
The Multipole Density Formalism (also referred to as Hansen-Coppens Formalism) is an X-ray crystallography method of electron density modelling proposed by Niels K. Hansen and Philip Coppens in 1978. Unlike the commonly used Independent Atom Mo ...
* Neutron diffraction
* Powder diffraction
* Ptychography
* Scherrer equation
The Scherrer equation, in X-ray diffraction and crystallography, is a formula that relates the size of sub-micrometre crystallites in a solid to the broadening of a peak in a diffraction pattern. It is often referred to, incorrectly, as a formula ...
* Small angle X-ray scattering (SAXS)
* Structure determination
* Ultrafast x-ray
* Wide angle X-ray scattering (WAXS)
References
Further reading
''International Tables for Crystallography''
*
*
*
Bound collections of articles
*
*
*
Textbooks
*
*
*
*
*
*
*
*
*
*
*
*
*
*
PDF copy of select chapters
*
*
*
Applied computational data analysis
*
Historical
*
*
*
*
*
*
*
External links
Tutorials
Simple, non technical introductionThe Crystallography Collection
video series from the Royal Institution
"Small Molecule Crystalization"
( PDF) at Illinois Institute of Technology
Illinois Institute of Technology (IIT) is a private research university in Chicago, Illinois. Tracing its history to 1890, the present name was adopted upon the merger of the Armour Institute and Lewis Institute in 1940. The university has pro ...
website
International Union of Crystallography
demonstrating properties of the diffraction pattern of a 2D crystal.
illustrating the relationship between crystal and diffraction pattern in 2D.
* ttp://nanohub.org/resources/5580 Online lecture on Modern X-ray Scattering Methods for Nanoscale Materials Analysisby Richard J. Matyi
Interactive Crystallography Timeline
from the Royal Institution
Primary databases
* Crystallography Open Database (COD)
Protein Data Bank
( PDB)
Nucleic Acid Databank
(NDB)
Cambridge Structural Database
( CSD)
Inorganic Crystal Structure Database
( ICSD)
Biological Macromolecule Crystallization Database
(BMCD)
Derivative databases
PDBsum
Proteopedia – the collaborative, 3D encyclopedia of proteins and other molecules
RNABase
HIC-Up database of PDB ligands
* Structural Classification of Proteins
The Structural Classification of Proteins (SCOP) database is a largely manual classification of protein structural domains based on similarities of their structures and amino acid sequences. A motivation for this classification is to determine t ...
database
* CATH Protein Structure Classification
List of transmembrane proteins with known 3D structure
* Orientations of Proteins in Membranes database
Structural validation
MolProbity structural validation suite
ProSA-web
NQ-Flipper
(check for unfavorable rotamers of Asn and Gln residues)
DALI server
(identifies proteins similar to a given protein)
{{DEFAULTSORT:X-Ray Crystallography
Laboratory techniques in condensed matter physics
Crystallography
Diffraction
Materials science
Protein structure
Protein methods
Protein imaging
Synchrotron-related techniques
Articles containing video clips
Crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wo ...
 X-ray crystallography is the experimental science determining the atomic and molecular structure of a
X-ray crystallography is the experimental science determining the atomic and molecular structure of a  Crystals, though long admired for their regularity and symmetry, were not investigated scientifically until the 17th century. Johannes Kepler hypothesized in his work ''Strena seu de Nive Sexangula'' (A New Year's Gift of Hexagonal Snow) (1611) that the hexagonal symmetry of snowflake crystals was due to a regular packing of spherical water particles.
Crystals, though long admired for their regularity and symmetry, were not investigated scientifically until the 17th century. Johannes Kepler hypothesized in his work ''Strena seu de Nive Sexangula'' (A New Year's Gift of Hexagonal Snow) (1611) that the hexagonal symmetry of snowflake crystals was due to a regular packing of spherical water particles.
 The Danish scientist Nicolas Steno (1669) pioneered experimental investigations of crystal symmetry. Steno showed that the angles between the faces are the same in every exemplar of a particular type of crystal, and René Just Haüy (1784) discovered that every face of a crystal can be described by simple stacking patterns of blocks of the same shape and size. Hence,
The Danish scientist Nicolas Steno (1669) pioneered experimental investigations of crystal symmetry. Steno showed that the angles between the faces are the same in every exemplar of a particular type of crystal, and René Just Haüy (1784) discovered that every face of a crystal can be described by simple stacking patterns of blocks of the same shape and size. Hence,  After Von Laue's pioneering research, the field developed rapidly, most notably by physicists William Lawrence Bragg and his father William Henry Bragg. In 1912–1913, the younger Bragg developed Bragg's law, which connects the observed scattering with reflections from evenly spaced planes within the crystal. The Braggs, father and son, shared the 1915 Nobel Prize in Physics for their work in crystallography. The earliest structures were generally simple and marked by one-dimensional symmetry. However, as computational and experimental methods improved over the next decades, it became feasible to deduce reliable atomic positions for more complicated two- and three-dimensional arrangements of atoms in the unit-cell.
The potential of X-ray crystallography for determining the structure of molecules and minerals—then only known vaguely from chemical and hydrodynamic experiments—was realized immediately. The earliest structures were simple inorganic crystals and minerals, but even these revealed fundamental laws of physics and chemistry. The first atomic-resolution structure to be "solved" (i.e., determined) in 1914 was that of table salt. The distribution of electrons in the table-salt structure showed that crystals are not necessarily composed of covalently bonded molecules, and proved the existence of ionic compounds. The structure of
After Von Laue's pioneering research, the field developed rapidly, most notably by physicists William Lawrence Bragg and his father William Henry Bragg. In 1912–1913, the younger Bragg developed Bragg's law, which connects the observed scattering with reflections from evenly spaced planes within the crystal. The Braggs, father and son, shared the 1915 Nobel Prize in Physics for their work in crystallography. The earliest structures were generally simple and marked by one-dimensional symmetry. However, as computational and experimental methods improved over the next decades, it became feasible to deduce reliable atomic positions for more complicated two- and three-dimensional arrangements of atoms in the unit-cell.
The potential of X-ray crystallography for determining the structure of molecules and minerals—then only known vaguely from chemical and hydrodynamic experiments—was realized immediately. The earliest structures were simple inorganic crystals and minerals, but even these revealed fundamental laws of physics and chemistry. The first atomic-resolution structure to be "solved" (i.e., determined) in 1914 was that of table salt. The distribution of electrons in the table-salt structure showed that crystals are not necessarily composed of covalently bonded molecules, and proved the existence of ionic compounds. The structure of  Since the 1920s, X-ray diffraction has been the principal method for determining the arrangement of atoms in minerals and
Since the 1920s, X-ray diffraction has been the principal method for determining the arrangement of atoms in minerals and  The first structure of an organic compound, hexamethylenetetramine, was solved in 1923. This was followed by several studies of long-chain
The first structure of an organic compound, hexamethylenetetramine, was solved in 1923. This was followed by several studies of long-chain  Crystal structures of proteins (which are irregular and hundreds of times larger than cholesterol) began to be solved in the late 1950s, beginning with the structure of
Crystal structures of proteins (which are irregular and hundreds of times larger than cholesterol) began to be solved in the late 1950s, beginning with the structure of  The oldest and most precise method of X-ray
The oldest and most precise method of X-ray  Although crystallography can be used to characterize the disorder in an impure or irregular crystal, crystallography generally requires a pure crystal of high regularity to solve the structure of a complicated arrangement of atoms. Pure, regular crystals can sometimes be obtained from natural or synthetic materials, such as samples of
Although crystallography can be used to characterize the disorder in an impure or irregular crystal, crystallography generally requires a pure crystal of high regularity to solve the structure of a complicated arrangement of atoms. Pure, regular crystals can sometimes be obtained from natural or synthetic materials, such as samples of  When a crystal is mounted and exposed to an intense beam of X-rays, it scatters the X-rays into a pattern of spots or ''reflections'' that can be observed on a screen behind the crystal. A similar pattern may be seen by shining a laser pointer at a
When a crystal is mounted and exposed to an intense beam of X-rays, it scatters the X-rays into a pattern of spots or ''reflections'' that can be observed on a screen behind the crystal. A similar pattern may be seen by shining a laser pointer at a  Having obtained initial phases, an initial model can be built. The atomic positions in the model and their respective Debye-Waller factors (or B-factors, accounting for the thermal motion of the atom) can be refined to fit the observed diffraction data, ideally yielding a better set of phases. A new model can then be fit to the new electron density map and successive rounds of refinement is carried out. This iterative process continues until the correlation between the diffraction data and the model is maximized. The agreement is measured by an ''R''-factor defined as
:
where ''F'' is the structure factor. A similar quality criterion is ''R''free, which is calculated from a subset (~10%) of reflections that were not included in the structure refinement. Both ''R'' factors depend on the resolution of the data. As a rule of thumb, ''R''free should be approximately the resolution in angstroms divided by 10; thus, a data-set with 2 Å resolution should yield a final ''R''free ~ 0.2. Chemical bonding features such as stereochemistry, hydrogen bonding and distribution of bond lengths and angles are complementary measures of the model quality. Phase bias is a serious problem in such iterative model building. ''Omit maps'' are a common technique used to check for this.
It may not be possible to observe every atom in the asymmetric unit. In many cases, Crystallographic disorder smears the electron density map. Weakly scattering atoms such as hydrogen are routinely invisible. It is also possible for a single atom to appear multiple times in an electron density map, e.g., if a protein sidechain has multiple (<4) allowed conformations. In still other cases, the crystallographer may detect that the covalent structure deduced for the molecule was incorrect, or changed. For example, proteins may be cleaved or undergo post-translational modifications that were not detected prior to the crystallization.
Having obtained initial phases, an initial model can be built. The atomic positions in the model and their respective Debye-Waller factors (or B-factors, accounting for the thermal motion of the atom) can be refined to fit the observed diffraction data, ideally yielding a better set of phases. A new model can then be fit to the new electron density map and successive rounds of refinement is carried out. This iterative process continues until the correlation between the diffraction data and the model is maximized. The agreement is measured by an ''R''-factor defined as
:
where ''F'' is the structure factor. A similar quality criterion is ''R''free, which is calculated from a subset (~10%) of reflections that were not included in the structure refinement. Both ''R'' factors depend on the resolution of the data. As a rule of thumb, ''R''free should be approximately the resolution in angstroms divided by 10; thus, a data-set with 2 Å resolution should yield a final ''R''free ~ 0.2. Chemical bonding features such as stereochemistry, hydrogen bonding and distribution of bond lengths and angles are complementary measures of the model quality. Phase bias is a serious problem in such iterative model building. ''Omit maps'' are a common technique used to check for this.
It may not be possible to observe every atom in the asymmetric unit. In many cases, Crystallographic disorder smears the electron density map. Weakly scattering atoms such as hydrogen are routinely invisible. It is also possible for a single atom to appear multiple times in an electron density map, e.g., if a protein sidechain has multiple (<4) allowed conformations. In still other cases, the crystallographer may detect that the covalent structure deduced for the molecule was incorrect, or changed. For example, proteins may be cleaved or undergo post-translational modifications that were not detected prior to the crystallization.
 Each X-ray diffraction image represents only a slice, a spherical slice of reciprocal space, as may be seen by the Ewald sphere construction. Both kout and kin have the same length, due to the elastic scattering, since the wavelength has not changed. Therefore, they may be represented as two radial vectors in a sphere in reciprocal space, which shows the values of q that are sampled in a given diffraction image. Since there is a slight spread in the incoming wavelengths of the incoming X-ray beam, the values of, ''F''(q), can be measured only for q vectors located between the two spheres corresponding to those radii. Therefore, to obtain a full set of Fourier transform data, it is necessary to rotate the crystal through slightly more than 180°, or sometimes less if sufficient symmetry is present. A full 360° rotation is not needed because of a symmetry intrinsic to the Fourier transforms of real functions (such as the electron density), but "slightly more" than 180° is needed to cover all of reciprocal space within a given resolution because of the curvature of the Ewald sphere. In practice, the crystal is rocked by a small amount (0.25–1°) to incorporate reflections near the boundaries of the spherical Ewald's shells.
Each X-ray diffraction image represents only a slice, a spherical slice of reciprocal space, as may be seen by the Ewald sphere construction. Both kout and kin have the same length, due to the elastic scattering, since the wavelength has not changed. Therefore, they may be represented as two radial vectors in a sphere in reciprocal space, which shows the values of q that are sampled in a given diffraction image. Since there is a slight spread in the incoming wavelengths of the incoming X-ray beam, the values of, ''F''(q), can be measured only for q vectors located between the two spheres corresponding to those radii. Therefore, to obtain a full set of Fourier transform data, it is necessary to rotate the crystal through slightly more than 180°, or sometimes less if sufficient symmetry is present. A full 360° rotation is not needed because of a symmetry intrinsic to the Fourier transforms of real functions (such as the electron density), but "slightly more" than 180° is needed to cover all of reciprocal space within a given resolution because of the curvature of the Ewald sphere. In practice, the crystal is rocked by a small amount (0.25–1°) to incorporate reflections near the boundaries of the spherical Ewald's shells.