|
Sodium Nitrate
Sodium nitrate is the chemical compound with the formula . This alkali metal nitrate salt is also known as Chile saltpeter (large deposits of which were historically mined in Chile) to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter. Sodium nitrate is a white deliquescent solid very soluble in water. It is a readily available source of the nitrate anion (NO3−), which is useful in several reactions carried out on industrial scales for the production of fertilizers, pyrotechnics, smoke bombs and other explosives, glass and pottery enamels, food preservatives (esp. meats), and solid rocket propellant. It has been mined extensively for these purposes. History The first shipment of saltpeter to Europe arrived in England from Peru in 1820 or 1825, right after that country's independence from Spain, but did not find any buyers and was dumped at sea in order to avoid customs toll.Friedrich Georg Wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal Nitrate
Alkali metal nitrates are chemical compounds consisting of an alkali metal (lithium, sodium, potassium, rubidium and caesium) and the nitrate ion. Only two are of major commercial value, the sodium and potassium salts. They are white, water-soluble salts with melting points ranging from 255 °C () to 414 °C () on a relatively narrow span of 159 °C only. The melting point of the alkali metal nitrates tends to increase from 255 °C to 414 °C (with an anomaly for rubidium being not properly aligned in the series) as the atomic mass and the ionic radius (naked cation) of the alkaline metal increases, going down in the column. Similarly, but not presented here in the table, the solubility of these salts in water also decreases with the atomic mass of the metal. Applications Sodium and potassium nitrates are commonly used as fertilizers. As they are also strong oxidizers, they enter pyrotechnic compositions and the manufacturing of explosives. A minor use is for coloring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is a non-Crystallinity, crystalline, often transparency and translucency, transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the Melting, molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silicon dioxide, silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term ''glass'', in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and glasses, eyeglasses, are so commonly made of silicate- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be * chemical energy, such as nitroglycerin or grain dust * pressurized gas, such as a gas cylinder, aerosol can, or BLEVE * nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smoke Bomb
A smoke bomb is a firework designed to produce a large amount of smoke upon ignition. History Early Japanese history saw the use of a rudimentary form of the smoke bomb. Explosives were common in Japan during the Mongol invasions of the 13th century. Soft cased hand-held bombs were later designed to release smoke, poison gas, and shrapnel made from iron and pottery. The modern smoke bomb was created in 1848, by the British inventor Robert Yale. He developed 17th-century Chinese-style fireworks and later modified the formula to produce more smoke for a longer period of time. Colored smoke devices use a formula that consists of an oxidizer (typically potassium nitrate, KNO3), a fuel (generally sugar), a moderator (such as sodium bicarbonate) to keep the reaction from getting too hot, and a powdered organic dye. The burning of this mixture boils the dye and forces it out of the device, where it condenses in the atmosphere to form a smoke of finely dispersed particles. Ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrotechnics
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, Pyrotechnic fastener, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. This trade relies upon self-contained and self-sustained exothermic chemical reactions to make heat, light, gas, smoke and/or sound. The name etymology, comes from the Greek words ''pyr'' ("fire") and ''tekhnikos'' ("made by art"). People responsible for the safe storage, handling, and functioning of pyrotechnic devices are known as pyrotechnicians. Proximate pyrotechnics Explosions, flashes, smoke, flames, fireworks and other pyrotechnic-driven effects used in the entertainment industry are referred to as proximate pyrotechnics. Proximate refers to the pyrotechnic device's location relative to an audience. In the majority of jurisdictions, special training and licensing must be obtained from local authorities to lega ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods. Historically fertilization came from natural or organic sources: compost, animal manure, human manure, harvested minerals, crop rotations and byproducts of human-nature industries (i.e. fish processing waste, or bloodmeal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
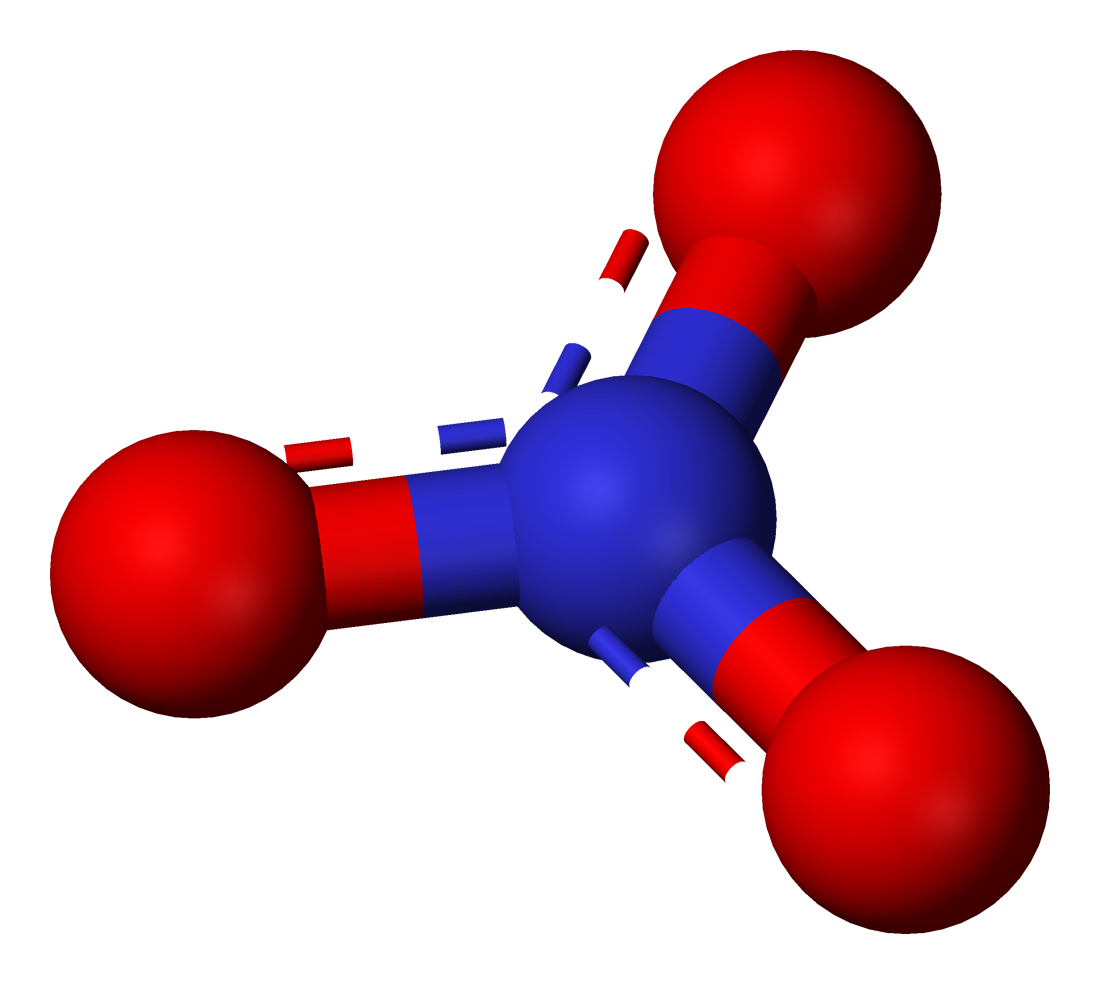
Nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures: Dietary nitrate A rich source of inorganic nitrate in the human diets come from leafy green foods, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niter
Niter or nitre is the mineral form of potassium nitrate, KNO3. It is a soft, white, highly soluble mineral found primarily in arid climates or cave deposits. Historically, the term ''niter'' was not well differentiated from natron, both of which have been very vaguely defined but generally refer to compounds of sodium or potassium joined with carbonate or nitrate ions. Characteristics Niter is a colorless to white mineral crystallizing in the orthorhombic crystal system. It is the mineral form of potassium nitrate, , and is soft (Mohs hardness 2), highly soluble in water, and easily fusible. Its crystal structure resembles that of aragonite, with potassium replacing calcium and nitrate replacing carbonate. It occurs in the soils of arid regions and as massive encrustations and efflorescent growths on cavern walls and ceilings where solutions containing alkali potassium and nitrate seep into the openings. It occasionally occurs as prismatic Acicular (crystal habit), acicular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitratine
Nitratine or nitratite, also known as cubic niter (UK: nitre), soda niter or Chile saltpeter (UK: Chile saltpetre), is a mineral, the naturally occurring form of sodium nitrate, NaNO3. Chemically it is the sodium analogue of saltpeter. Nitratine crystallizes in the trigonal system, but rarely occurs as well formed crystals. It is isostructural with calcite. It is quite soft and light with a Mohs hardness of 1.5 to 2 and a specific gravity of 2.24 to 2.29. Its refractive indices are nω=1.587 and nε=1.336. The typical form is as coatings of white, grey to yellowish brown masses. The rare crystals when found typically have the scalenohedral form of the calcite structure. It is found only as an efflorescence in very dry environments. It is very soluble in water such that it is deliquescent and will absorb water out of the air and turn into a ''puddle'' of sodium nitrate solution when exposed to humid air. Nitratine was once an important source of nitrates for fertilizer a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)