|
Multipole Density Formalism
The Multipole Density Formalism (also referred to as Hansen-Coppens Formalism) is an X-ray crystallography method of electron density modelling proposed by Niels K. Hansen and Philip Coppens in 1978. Unlike the commonly used Independent Atom Model, the Hansen-Coppens Formalism presents an aspherical approach, allowing one to model the electron distribution around a nucleus separately in different directions and therefore describe numerous chemical features of a molecule inside the unit cell of an examined crystal in detail. Theory Independent Atom Model The Independent Atom Model (abbreviated to IAM), upon which the Multipole Model is based, is a method of charge density modelling. It relies on an assumption that electron distribution around the atom is isotropic, and that therefore charge density is dependent only on the distance from a nucleus. The choice of the radial function used to describe this electron density is arbitrary, granted that its value at the origin is f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philip Coppens (chemist)
Philip Coppens (October 24, 1930 – June 21, 2017) was a Dutch-born American chemist and crystallographer known for his work on charge density analysis using X-rays crystallography and the pioneering work in the field of photocrystallography. Education and career The Amersfoort-born Coppens received his B.S. and Ph.D. degrees from the University of Amsterdam in 1954 and 1960, where he was supervised by Carolina MacGillavry. In 1968, following appointments at the Weizmann Institute and Brookhaven National Laboratory, he was appointed in the chemistry department at the State University of New York at Buffalo. He was a SUNY Distinguished Professor and holder of the Henry M. Woodburn Chair of Chemistry. Among the many 3-dimensional structures Coppens characterized is the nitroprusside ion. Honours and awards Coppens was a corresponding member of the Royal Netherlands Academy of Arts and Sciences since 1979 and a fellow of the American Association for the Advancement of Scie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
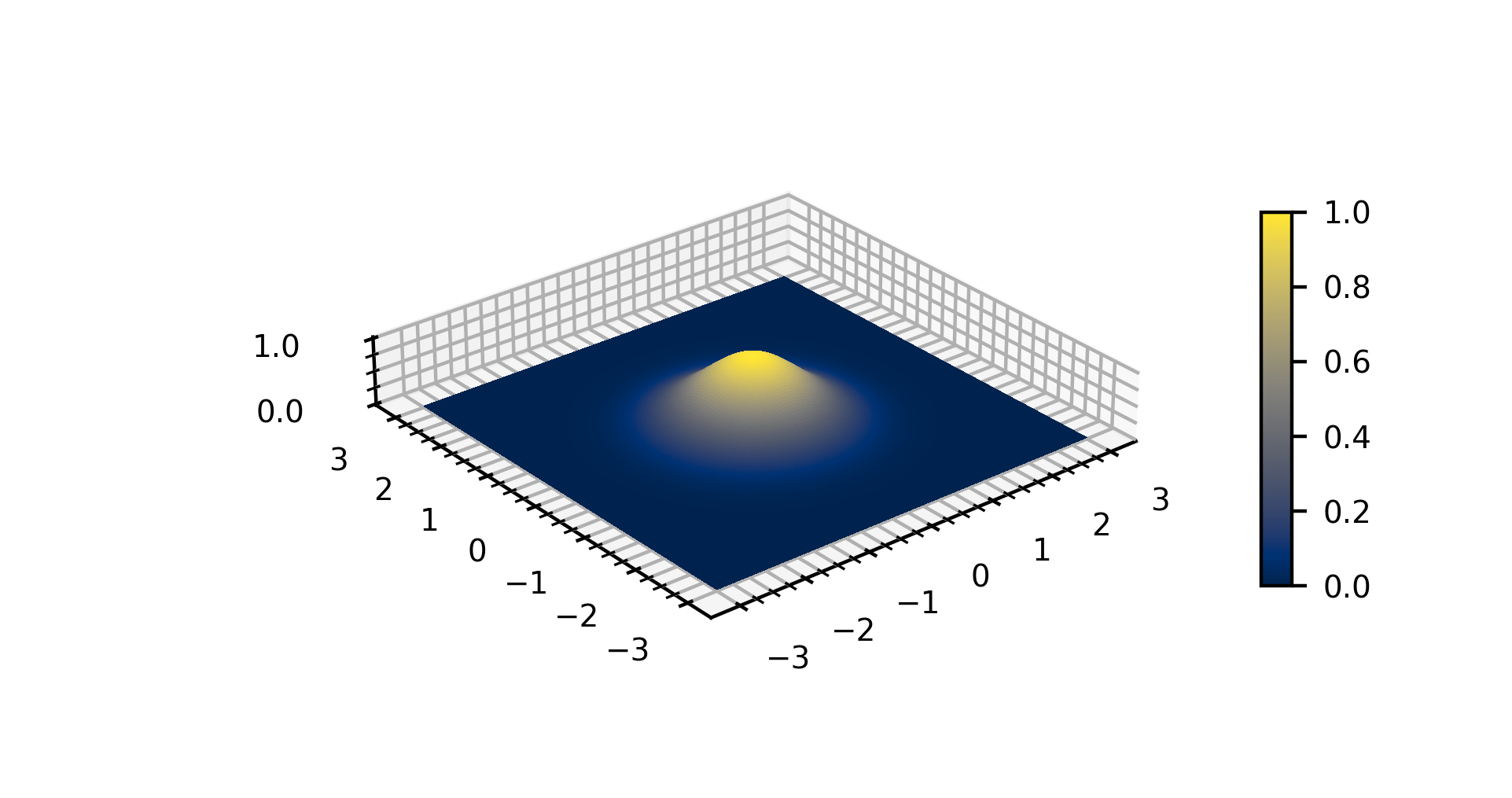
Gaussian Function
In mathematics, a Gaussian function, often simply referred to as a Gaussian, is a function of the base form f(x) = \exp (-x^2) and with parametric extension f(x) = a \exp\left( -\frac \right) for arbitrary real constants , and non-zero . It is named after the mathematician Carl Friedrich Gauss. The graph of a Gaussian is a characteristic symmetric " bell curve" shape. The parameter is the height of the curve's peak, is the position of the center of the peak, and (the standard deviation, sometimes called the Gaussian RMS width) controls the width of the "bell". Gaussian functions are often used to represent the probability density function of a normally distributed random variable with expected value and variance . In this case, the Gaussian is of the form g(x) = \frac \exp\left( -\frac \frac \right). Gaussian functions are widely used in statistics to describe the normal distributions, in signal processing to define Gaussian filters, in image processing where two-di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Bond Theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule. History Lothar Meyer in his 1864 book, ''Die modernen Theorien der Chemie'', contained an early version of the periodic table containing 28 elements, classified elements into six families by their valence—for the first time, elements had been grouped according to their valence. Works on organizing the elements by atomic weight, until then had been stymied by the widespread use of equivalent weights for the elements, rather than atomic weights. In 1916, G. N. Lewis proposed that a chemical bond forms by the interaction of two shared bonding electrons, with the repr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Coordinates
Local coordinates are the ones used in a ''local coordinate system'' or a ''local coordinate space''. Simple examples: * Houses. In order to work in a house construction, the measurements are referred to a control arbitrary point that will allow to check it: stick/sticks on the ground, steel bar, nails... * Addresses. Using house numbers to locate a house on a street; the street is a local coordinate system within a larger system composed of city townships, states, countries, postal codes, etc. Local systems exist for convenience. On ancient times, every work was made on relative bases as there was no conception of global systems. Practically, it is better to use local systems for small works as houses, buildings... For most of the applications, it is desired the position of one element relative to one building or location, and in a more local way, relative to one furniture or person. In a regular way, you will not give your position by geographical coordinates rather than "I am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordinate System
In geometry, a coordinate system is a system that uses one or more numbers, or coordinates, to uniquely determine the position of the points or other geometric elements on a manifold such as Euclidean space. The order of the coordinates is significant, and they are sometimes identified by their position in an ordered tuple and sometimes by a letter, as in "the ''x''-coordinate". The coordinates are taken to be real numbers in elementary mathematics, but may be complex numbers or elements of a more abstract system such as a commutative ring. The use of a coordinate system allows problems in geometry to be translated into problems about numbers and ''vice versa''; this is the basis of analytic geometry. Common coordinate systems Number line The simplest example of a coordinate system is the identification of points on a line with real numbers using the '' number line''. In this system, an arbitrary point ''O'' (the ''origin'') is chosen on a given line. The coordinate of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spherical Harmonics
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields. Since the spherical harmonics form a complete set of orthogonal functions and thus an orthonormal basis, each function defined on the surface of a sphere can be written as a sum of these spherical harmonics. This is similar to periodic functions defined on a circle that can be expressed as a sum of circular functions (sines and cosines) via Fourier series. Like the sines and cosines in Fourier series, the spherical harmonics may be organized by (spatial) angular frequency, as seen in the rows of functions in the illustration on the right. Further, spherical harmonics are basis functions for irreducible representations of SO(3), the group of rotations in three dimensions, and thus play a central role in the group theoretic discussion of SO(3). Spherical harmonics originate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler substances by any chemical reaction. The number of protons in the nucleus is the defining property of an element, and is referred to as its atomic number (represented by the symbol ''Z'') – all atoms with the same atomic number are atoms of the same element. Almost all of the baryonic matter of the universe is composed of chemical elements (among rare exceptions are neutron stars). When different elements undergo chemical reactions, atoms are rearranged into new compounds held together by chemical bonds. Only a minority of elements, such as silver and gold, are found uncombined as relatively pure native element minerals. Nearly all other naturally occurring elements occur in the Earth as compounds or mixtures. Air is primarily a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Normalization (statistics)
In statistics and applications of statistics, normalization can have a range of meanings. In the simplest cases, normalization of ratings means adjusting values measured on different scales to a notionally common scale, often prior to averaging. In more complicated cases, normalization may refer to more sophisticated adjustments where the intention is to bring the entire probability distributions of adjusted values into alignment. In the case of normalization of scores in educational assessment, there may be an intention to align distributions to a normal distribution. A different approach to normalization of probability distributions is quantile normalization, where the quantiles of the different measures are brought into alignment. In another usage in statistics, normalization refers to the creation of shifted and scaled versions of statistics, where the intention is that these normalized values allow the comparison of corresponding normalized values for different datasets i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Charge
A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+. Partial charges are created due to the asymmetric distribution of electrons in chemical bonds. For example, in a polar covalent bond like HCl, the shared electron oscillates between the bonded atoms. The resulting partial charges are a property only of zones within the distribution, and not the assemblage as a whole. For example, chemists often choose to look at a small space surrounding the nucleus of an atom: When an electrically neutral atom bonds chemically to another neutral atom that is more electronegative, its electrons are partially drawn away. This leaves the region about that atom's nucleus with a partial positive charge, and it creates a partial negative charge on the atom to which it is bonded. In such a situation, the distributed charges taken ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Independence
In the theory of vector spaces, a set of vectors is said to be if there is a nontrivial linear combination of the vectors that equals the zero vector. If no such linear combination exists, then the vectors are said to be . These concepts are central to the definition of dimension. A vector space can be of finite dimension or infinite dimension depending on the maximum number of linearly independent vectors. The definition of linear dependence and the ability to determine whether a subset of vectors in a vector space is linearly dependent are central to determining the dimension of a vector space. Definition A sequence of vectors \mathbf_1, \mathbf_2, \dots, \mathbf_k from a vector space is said to be ''linearly dependent'', if there exist scalars a_1, a_2, \dots, a_k, not all zero, such that :a_1\mathbf_1 + a_2\mathbf_2 + \cdots + a_k\mathbf_k = \mathbf, where \mathbf denotes the zero vector. This implies that at least one of the scalars is nonzero, say a_1\ne 0, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


%2C_black_and_white.png)
