3.png) |
Bond Stretch Isomer
In chemistry, bond stretch isomerism is a concept of isomerism based on variations of bond length. The concept was proposed in the 1970s but was refuted in the 1990s. The phenomenon was first invoked to explain the observation of blue and green isomers of ''mer''-MoOCl2(PMe2Ph)3, where PMe2Ph is dimethylphenylphosphine. These isomers were shown, purportedly, by X-ray crystallography to differ with respect to the length of the Mo-O bond, which differed by 0.2 Å. Subsequent work showed that the supposed green bond stretch isomer consisted of blue ''mer''-MoOCl2(PMe2Ph)3 contaminated with a small amount of yellow ''mer''-MoCl3(PMe2Ph)3. The nearly isomorphous replacement of Mo-O unit with small amounts of Mo-Cl unit results in artifactually long Mo-O distance in the green sample. In essence the deception arises because the crystallographic disorder was not modeled appropriately. Several such examples were uncovered. Special examples Bond stretch isomerism is confirmed for compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work (pharmacology), and how to collect DNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Isomerism
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Dimethylphenylphosphine
Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. It is colorless air sensitive liquid. It is a member of series (CH3)3-n(C6H5)2P that also includes n = 0, n = 2, and n = 3 that are often employed as ligands in metal phosphine complexes. Preparation Dimethylphenylphosphine is prepared by the reaction of methylmagnesium halide with dichlorophenylphosphine. :(C6H5)Cl2P + 2CH3MgBr → (C6H5)(CH3)2P + 2MgBrCl The phosphine is purified by distillation under reduced pressure. A solution of (C6H5)(CH3)2P in CDCl3 shows proton NMR signals at δ 7.0-7.5 and a doublet at δ 1.2. The phosphorus-31 NMR spectrum shows a singlet at -45.9 ppm in CDCl3. Structure and properties Dimethylphenylphosphine is a pyramidal molecule where the phenyl group and two methyl groups are connected to the phosphorus. The bond length and angle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Crystallographic Disorder
In X-ray crystallography, crystallographic disorder describes the cocrystallization of more than one rotamer, conformer, or isomer In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. ... where the center of mass of each form is identical or unresolvable. As a consequence of disorder, the crystallographic solution is the sum of the various forms. In many cases, the components of the disorder are equally abundant, and, in other cases, the weighting coefficients for each component differ. Disorder can entail a pair or several components. Disorder usually arises when the forms are nearly equal in energy and the crystal lattice is sufficiently spacious to accommodate the various components. File:DOSBIW.png, Structure of Mo(CH3)5 showing 4-fold disorder of one methyl group.{{cite journal, jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
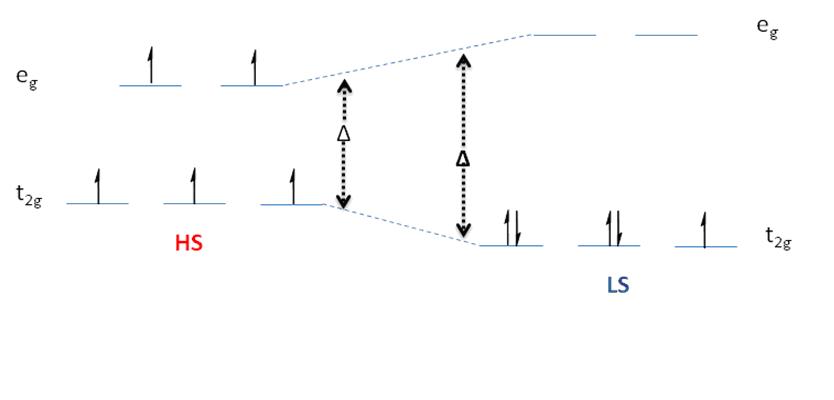 |
Spin Crossover
Spin crossover (SCO) is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to an external stimulus. The stimuli can include temperature or pressure. Spin crossover is sometimes referred to as spin transition or spin equilibrium behavior. The change in spin state usually involves interchange of low spin (LS) and high spin (HS) configuration. Spin crossover is commonly observed with first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves typically plot the high-spin molar fraction against temperature. Often a gradual spin transition is followed by an abrupt (ΔT = 10K) transition with hysteresis and a two-step transition. The abruptness with hysteresis indicates cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Pentamethylcyclopentadienyl Ruthenium Dichloride Dimer
Pentamethylcyclopentadienyl ruthenium dichloride is an organoruthenium chemistry with the formula C5(CH3)5)RuCl2sub>2, commonly abbreviated p*RuCl2sub>2. This brown paramagnetic solid is a reagent in organometallic chemistry. It is an unusual example of a compound that exists as isomers that differ in the intermetallic separation, a difference that is manifested in a number of physical properties. Preparation, structure, reactions The compound has C2h symmetry, with each metal atom having pseudo-octahedral geometry. In the crystal structure, two isomers are observed in the unit cell, one with a 2.93 Å ruthenium–ruthenium bond and the other with a long internuclear distance of 3.75 Å. The former isomer is diamagnetic, and the latter is magnetic. It is prepared by the reaction of hydrated ruthenium trichloride with pentamethylcyclopentadiene. :2 Cp*H + 2 RuCl3·3H2O → p*RuCl2sub>2 + 2 HCl + 6 H2O The reaction is accompanied by formation of decamethylruthenocen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |