production of hydrogen on:
[Wikipedia]
[Google]
[Amazon]
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from
 There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. Carbon dioxide can be separated from natural gas with a 70–85% efficiency for hydrogen production and from other hydrocarbons to varying degrees of efficiency. Specifically, bulk hydrogen is usually produced by the steam reforming of methane or natural gas.
There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. Carbon dioxide can be separated from natural gas with a 70–85% efficiency for hydrogen production and from other hydrocarbons to varying degrees of efficiency. Specifically, bulk hydrogen is usually produced by the steam reforming of methane or natural gas.
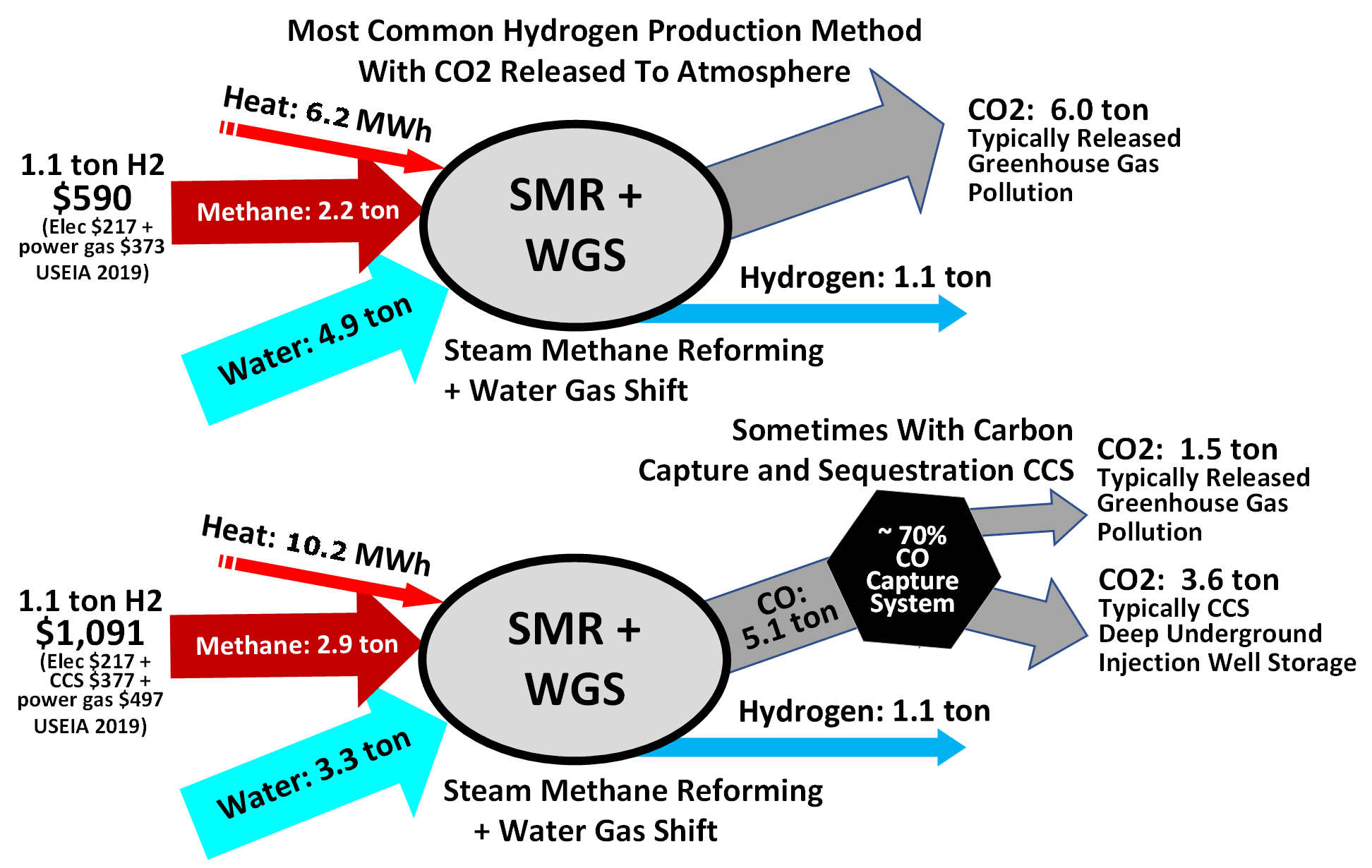 For this process, high temperature steam (H2O) reacts with methane (CH4) in an endothermic reaction to yield
For this process, high temperature steam (H2O) reacts with methane (CH4) in an endothermic reaction to yield
 Pyrolysis of methane is a hydrogen production process from natural gas. Hydrogen separation occurs in one step via flow through a molten metal catalyst in a "
Pyrolysis of methane is a hydrogen production process from natural gas. Hydrogen separation occurs in one step via flow through a molten metal catalyst in a "
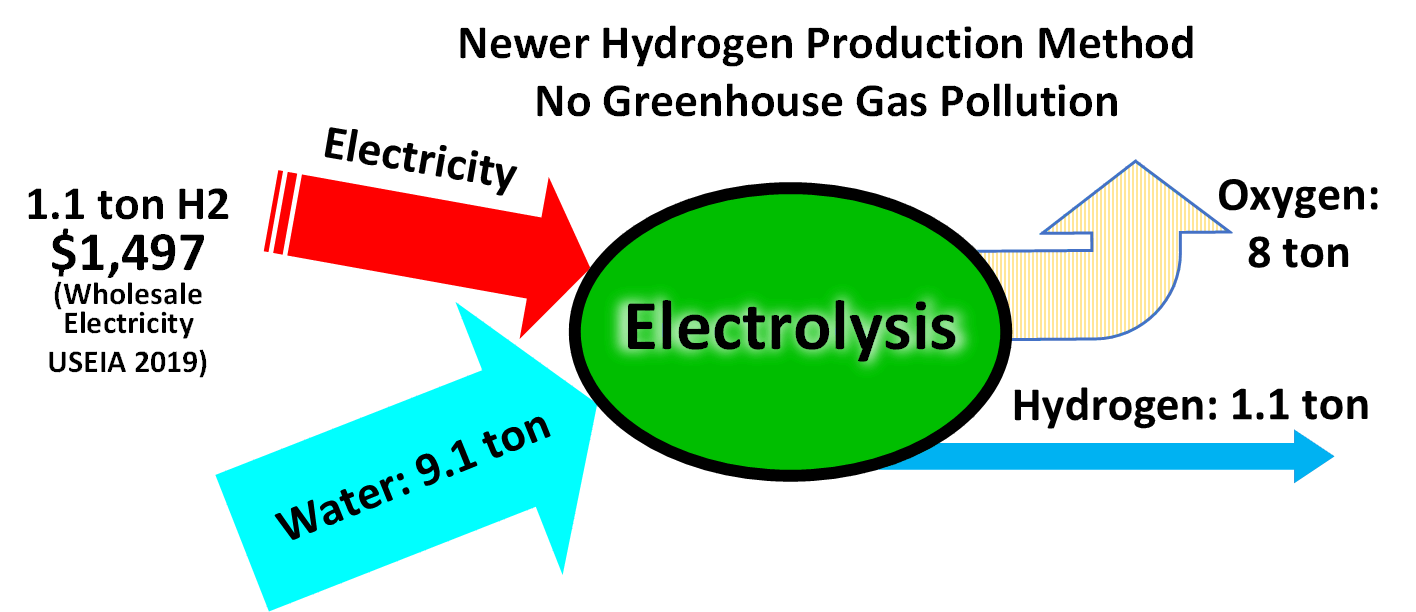 Around 8 GW of electrolysis capacity is installed worldwide in 2020, accounting for around 4% of global hydrogen production.
Electrolysis consists of using electricity to split water into hydrogen and oxygen. Electrolysis of water is 70–80% efficient (a 20–30% conversion loss) while steam reforming of natural gas has a thermal efficiency between 70 and 85%. The electrical efficiency of electrolysis is expected to reach 82–86% before 2030, while also maintaining durability as progress in this area continues apace.
Water electrolysis can operate between , while steam methane reforming requires temperatures between . The difference between the two methods is the primary energy used; either electricity (for electrolysis) or natural gas (for steam methane reforming). Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community. With the objective of reducing the cost of hydrogen production, renewable sources of energy have been targeted to allow electrolysis.
There are three main types of electrolytic cells, solid oxide electrolyser cells (SOECs), polymer electrolyte membrane cells (PEM) and
Around 8 GW of electrolysis capacity is installed worldwide in 2020, accounting for around 4% of global hydrogen production.
Electrolysis consists of using electricity to split water into hydrogen and oxygen. Electrolysis of water is 70–80% efficient (a 20–30% conversion loss) while steam reforming of natural gas has a thermal efficiency between 70 and 85%. The electrical efficiency of electrolysis is expected to reach 82–86% before 2030, while also maintaining durability as progress in this area continues apace.
Water electrolysis can operate between , while steam methane reforming requires temperatures between . The difference between the two methods is the primary energy used; either electricity (for electrolysis) or natural gas (for steam methane reforming). Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community. With the objective of reducing the cost of hydrogen production, renewable sources of energy have been targeted to allow electrolysis.
There are three main types of electrolytic cells, solid oxide electrolyser cells (SOECs), polymer electrolyte membrane cells (PEM) and
 As of 2020, the cost of hydrogen by electrolysis is around $3–8/kg. Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–82%, producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity. At an electricity cost of $0.06/kWh, as set out in the Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions. The report by IRENA.ORG is an extensive factual report of present-day industrial hydrogen production consuming about 53 to 70 kWh per kg could go down to about 45 kWh/kg . The thermodynamic energy required for hydrogen by electrolysis translates to 33 kWh/kg, which is higher than steam reforming with carbon capture and higher than methane pyrolysis.
One of the advantages of electrolysis over hydrogen from steam methane reforming (SMR) is that the hydrogen can be produced on-site, meaning that the costly process of delivery via truck or pipeline is avoided.
Steam methane reforming is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis.
As of 2020, the cost of hydrogen by electrolysis is around $3–8/kg. Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–82%, producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity. At an electricity cost of $0.06/kWh, as set out in the Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions. The report by IRENA.ORG is an extensive factual report of present-day industrial hydrogen production consuming about 53 to 70 kWh per kg could go down to about 45 kWh/kg . The thermodynamic energy required for hydrogen by electrolysis translates to 33 kWh/kg, which is higher than steam reforming with carbon capture and higher than methane pyrolysis.
One of the advantages of electrolysis over hydrogen from steam methane reforming (SMR) is that the hydrogen can be produced on-site, meaning that the costly process of delivery via truck or pipeline is avoided.
Steam methane reforming is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis.
 Biological hydrogen can be produced in an
Biological hydrogen can be produced in an
 Besides dark fermentation, electrohydrogenesis (electrolysis using microbes) is another possibility. Using microbial fuel cells, wastewater or plants can be used to generate power. Biocatalysed electrolysis should not be confused with biological hydrogen production, as the latter only uses algae and with the latter, the algae itself generates the hydrogen instantly, where with biocatalysed electrolysis, this happens after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.
Besides dark fermentation, electrohydrogenesis (electrolysis using microbes) is another possibility. Using microbial fuel cells, wastewater or plants can be used to generate power. Biocatalysed electrolysis should not be confused with biological hydrogen production, as the latter only uses algae and with the latter, the algae itself generates the hydrogen instantly, where with biocatalysed electrolysis, this happens after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.

fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
s by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification Coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and water vapour (H2O)—from coal and water, air and/or oxygen.
Historically, coal ...
. Other methods of hydrogen production include biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms bi ...
gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
, zero-CO2-emission methane pyrolysis, and electrolysis of water. The latter processes, methane pyrolysis as well as water electrolysis can be done directly with any source of electricity, such as solar power.
The production of hydrogen plays a key role in any industrialized society
Industrialisation ( alternatively spelled industrialization) is the period of social and economic change that transforms a human group from an agrarian society into an industrial society. This involves an extensive re-organisation of an econo ...
, since hydrogen is required for many essential chemical processes. In 2020, roughly 87 million tons of hydrogen was produced worldwide for various uses, such as oil refining
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefie ...
, and in the production of ammonia (NH3) (through the Haber process) and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
(CH3OH) (through reduction of carbon monoxide O, and also as a fuel in transportation. The global hydrogen generation market was valued at US$135.94 billion in 2021, and expected to grow to US$219.2 billion by 2030, with a compound annual growth rate (CAGR) of 5.4% from 2021 to 2030.
Methods of hydrogen production
 There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. Carbon dioxide can be separated from natural gas with a 70–85% efficiency for hydrogen production and from other hydrocarbons to varying degrees of efficiency. Specifically, bulk hydrogen is usually produced by the steam reforming of methane or natural gas.
There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. Carbon dioxide can be separated from natural gas with a 70–85% efficiency for hydrogen production and from other hydrocarbons to varying degrees of efficiency. Specifically, bulk hydrogen is usually produced by the steam reforming of methane or natural gas.
Steam methane reforming
Steam methane reforming (SMR) is a method of producing hydrogen from natural gas, which is mostly methane (CH4). It is currently the cheapest source of industrial hydrogen. Nearly 50% of the world's hydrogen is being produced by this method. The process consists of heating the gas to between in the presence of steam and a nickel catalyst. The resulting endothermic reaction breaks up the methane molecules and forms carbon monoxide and molecular hydrogen (H2). The carbon monoxide gas can then be passed with steam overiron oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of whic ...
or other oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s and undergo a water-gas shift reaction to obtain further quantities of H2. The downside to this process is that its byproducts are major atmospheric release of CO2, CO and other greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
es. Depending on the quality of the feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials that are feedstock for future finished products. As feedst ...
(natural gas, rich gas
Rich may refer to:
Common uses
* Rich, an entity possessing wealth
* Rich, an intense flavor, color, sound, texture, or feeling
** Rich (wine), a descriptor in wine tasting
Places United States
* Rich, Mississippi, an unincorporated commun ...
es, naphtha, etc.), one ton of hydrogen produced will also produce 9 to 12 tons of CO2, a greenhouse gas that may be captured.
 For this process, high temperature steam (H2O) reacts with methane (CH4) in an endothermic reaction to yield
For this process, high temperature steam (H2O) reacts with methane (CH4) in an endothermic reaction to yield syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used as ...
.
:CH4 + H2O → CO + 3 H2
In a second stage, additional hydrogen is generated through the lower-temperature, exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
, water-gas shift reaction, performed at about :
:CO + H2O → CO2 + H2
Essentially, the oxygen (O) atom is stripped from the additional water (steam) to oxidize CO to CO2. This oxidation also provides energy to maintain the reaction. Additional heat required to drive the process is generally supplied by burning some portion of the methane.
Other fossil fuel methods
Methane pyrolysis
 Pyrolysis of methane is a hydrogen production process from natural gas. Hydrogen separation occurs in one step via flow through a molten metal catalyst in a "
Pyrolysis of methane is a hydrogen production process from natural gas. Hydrogen separation occurs in one step via flow through a molten metal catalyst in a "bubble column
Bubble, Bubbles or The Bubble may refer to:
Common uses
* Bubble (physics), a globule of one substance in another, usually gas in a liquid
** Soap bubble
* Economic bubble, a situation where asset prices are much higher than underlying fundame ...
". It is a "no-greenhouse-gas" approach for potentially low-cost hydrogen production being measured for its capability to scale up and for operation at scale.
The process is conducted at higher temperatures (1065 °C or 1950 °F). Other forms of methane pyrolysis, such as the thermo-catalytic decomposition of methane, however, are able to operate at a reduced temperature between 600 °C - 1000 °C depending on the chosen catalyst.
: (g) → C(s) + 2 (g) ΔH° = 74.8 kJ/mol
The joule per mole (symbol: J·mol−1 or J/mol) is the unit of energy per amount of substance in the International System of Units (SI), such that energy is measured in joules, and the amount of substance is measured in moles.
It is also an SI ...
The industrial quality solid carbon can then be sold as manufacturing feedstock or landfill
A landfill site, also known as a tip, dump, rubbish dump, garbage dump, or dumping ground, is a site for the disposal of waste materials. Landfill is the oldest and most common form of waste disposal, although the systematic burial of the waste ...
ed, it is not released into the atmosphere and does not pollute groundwater in landfills.
Partial oxidation
Hydrogen production from heavy hydrocarbons, which are unsuitable for catalytic steam reforming, is achieved by partial oxidation. A fuel-air or fuel-oxygen mixture is partially combusted, resulting in a hydrogen- and carbon monoxide-rich syngas. More hydrogen and carbon dioxide are then obtained from carbon monoxide (and water) via the water-gas shift reaction. Carbon dioxide can be co-fed to lower the hydrogen to carbon monoxide ratio. The partial oxidation reaction occurs when a substoichiometric fuel-air mixture or fuel-oxygen is partially combusted in a reformer or partial oxidation reactor. A distinction is made between ''thermal'' partial oxidation (TPOX) and ''catalytic'' partial oxidation (CPOX). The chemical reaction takes the general form: :C''n''H''m'' + ''n''/2 O2 → ''n'' CO + ''m''/2 H2 Idealized examples forheating oil
Heating oil is any petroleum product or other oil used for heating; a fuel oil. Most commonly, it refers to low viscosity grades of fuel oil used for furnaces or boilers use for home heating and in other buildings. Home heating oil is often a ...
and coal, assuming compositions C12H24 and C24H12 respectively, are as follows:
:C12H24 + 6 O2 → 12 CO + 12 H2
:C24H12 + 12 O2 → 24 CO + 6 H2
Plasma reforming
TheKværner process The Kværner process or the Kværner carbon black and hydrogen process (CB&H) is a method of producing carbon black and hydrogen gas from hydrocarbons such as methane, natural gas and biogas with no greenhouse gas pollution. The process was develope ...
or Kvaerner carbon black & hydrogen process (CB&H) is a plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
reforming method, developed in the 1980s by a Norwegian company of the same name, for the production of hydrogen and carbon black from liquid hydrocarbons (CnHm). Of the available energy of the feed, approximately 48% is contained in the hydrogen, 40% is contained in activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
and 10% in superheated steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.
Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its ...
. CO2 is not produced in the process.
A variation of this process is presented in 2009 using, plasma arc waste disposal technology for the production of hydrogen, heat and carbon from methane and natural gas in a plasma converter.
Coal
For the production of hydrogen from coal,coal gasification Coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and water vapour (H2O)—from coal and water, air and/or oxygen.
Historically, coal ...
is used. The process of coal gasification uses steam and oxygen to break molecular bonds in coal and form a gaseous mixture of hydrogen and carbon monoxide.Hordeski, M. F. Alternative fuels: the future of hydrogen. 171-199 (The Fairmont Press, inc., 2007). Carbon dioxide and pollutants may be more easily removed from gas obtained from coal gasification versus coal combustion. Another method for conversion is low-temperature and high-temperature coal carbonization.
Coke oven gas made from pyrolysis (oxygen free heating) of coal has about 60% hydrogen, the rest being methane, carbon monoxide, carbon dioxide, ammonia, molecular nitrogen, and hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S). Hydrogen can be separated from other impurities by the pressure-swing adsorption process. Japanese steel companies have carried out production of hydrogen by this method.
Petroleum coke
Petroleum coke can also be converted to hydrogen-richsyngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principly used for producing ammonia or methanol. Syngas is combustible and can be used as ...
via coal gasification. The produced syngas consists mainly of hydrogen, carbon monoxide and H2S from the sulfur in the coke feed. Gasification is an option for producing hydrogen from almost any carbon source.
Depleted oil wells
Injecting appropriate microbes into depleted oils allows them to extract hydrogen from the remaining, unrecoverable oil. Since the only inputs are the microbes, production costs are low. The method also produces concentrated that needs to be captured and stored.From water
Methods to produce hydrogen without the use of fossil fuels involve the process of water splitting, or splitting the water molecule (H2O) into its components oxygen and hydrogen. When the source of energy for water splitting is renewable or low-carbon, the hydrogen produced is sometimes referred to as green hydrogen. The conversion can be accomplished in several ways, but all methods are generally more expensive than fossil-fuel based production methods.Electrolysis
 Around 8 GW of electrolysis capacity is installed worldwide in 2020, accounting for around 4% of global hydrogen production.
Electrolysis consists of using electricity to split water into hydrogen and oxygen. Electrolysis of water is 70–80% efficient (a 20–30% conversion loss) while steam reforming of natural gas has a thermal efficiency between 70 and 85%. The electrical efficiency of electrolysis is expected to reach 82–86% before 2030, while also maintaining durability as progress in this area continues apace.
Water electrolysis can operate between , while steam methane reforming requires temperatures between . The difference between the two methods is the primary energy used; either electricity (for electrolysis) or natural gas (for steam methane reforming). Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community. With the objective of reducing the cost of hydrogen production, renewable sources of energy have been targeted to allow electrolysis.
There are three main types of electrolytic cells, solid oxide electrolyser cells (SOECs), polymer electrolyte membrane cells (PEM) and
Around 8 GW of electrolysis capacity is installed worldwide in 2020, accounting for around 4% of global hydrogen production.
Electrolysis consists of using electricity to split water into hydrogen and oxygen. Electrolysis of water is 70–80% efficient (a 20–30% conversion loss) while steam reforming of natural gas has a thermal efficiency between 70 and 85%. The electrical efficiency of electrolysis is expected to reach 82–86% before 2030, while also maintaining durability as progress in this area continues apace.
Water electrolysis can operate between , while steam methane reforming requires temperatures between . The difference between the two methods is the primary energy used; either electricity (for electrolysis) or natural gas (for steam methane reforming). Due to their use of water, a readily available resource, electrolysis and similar water-splitting methods have attracted the interest of the scientific community. With the objective of reducing the cost of hydrogen production, renewable sources of energy have been targeted to allow electrolysis.
There are three main types of electrolytic cells, solid oxide electrolyser cells (SOECs), polymer electrolyte membrane cells (PEM) and alkaline electrolysis cell
Alkaline water electrolysis has a long history in the chemical industry. It is a type of electrolyzer that is characterized by having two electrodes operating in a liquid alkaline electrolyte solution of potassium hydroxide (KOH) or sodium hydroxi ...
s (AECs). Traditionally, alkaline electrolysers are cheaper in terms of investment (they generally use nickel catalysts), but less-efficient; PEM electrolysers, conversely, are more expensive (they generally use expensive platinum group metal catalysts) but are more efficient and can operate at higher current densities, and can therefore be possibly cheaper if the hydrogen production is large enough.
SOECs operate at high temperatures, typically around . At these high temperatures, a significant amount of the energy required can be provided as thermal energy (heat), and as such is termed high-temperature electrolysis. The heat energy can be provided from a number of different sources, including waste industrial heat, nuclear power stations or concentrated solar thermal plants. This has the potential to reduce the overall cost of the hydrogen produced by reducing the amount of electrical energy required for electrolysis.
PEM electrolysis cells typically operate below . These cells have the advantage of being comparatively simple and can be designed to accept widely varying voltage inputs, which makes them ideal for use with renewable sources of energy such as photovoltaic solar panels. AECs optimally operate at high concentrations of electrolyte (KOH or potassium carbonate) and at high temperatures, often near .
Industrial output and efficiency
Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m3), assumingstandard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
of the H2. The lower the energy used by a generator, the higher would be its efficiency; a 100%-efficient electrolyser would consume of hydrogen, . Practical electrolysis typically uses a rotating electrolyser, where centrifugal force helps separate gas bubbles from water. Such an electrolyser at 15 bar pressure may consume , and a further if the hydrogen is compressed for use in hydrogen cars.
Conventional alkaline electrolysis has an efficiency of about 70%, however advanced alkaline water electrolyser with efficiency of up to 82% are available. Accounting for the use of the higher heat value (because inefficiency via heat can be redirected back into the system to create the steam required by the catalyst), average working efficiencies for PEM electrolysis are around 80%, or 82% using the most modern alkaline electrolysers.
PEM efficiency is expected to increase to approximately 86% before 2030. Theoretical efficiency for PEM electrolysers is predicted up to 94%.
 As of 2020, the cost of hydrogen by electrolysis is around $3–8/kg. Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–82%, producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity. At an electricity cost of $0.06/kWh, as set out in the Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions. The report by IRENA.ORG is an extensive factual report of present-day industrial hydrogen production consuming about 53 to 70 kWh per kg could go down to about 45 kWh/kg . The thermodynamic energy required for hydrogen by electrolysis translates to 33 kWh/kg, which is higher than steam reforming with carbon capture and higher than methane pyrolysis.
One of the advantages of electrolysis over hydrogen from steam methane reforming (SMR) is that the hydrogen can be produced on-site, meaning that the costly process of delivery via truck or pipeline is avoided.
Steam methane reforming is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis.
As of 2020, the cost of hydrogen by electrolysis is around $3–8/kg. Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–82%, producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg or about 40 kWh/kg) requires 50–55 kWh of electricity. At an electricity cost of $0.06/kWh, as set out in the Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg.
The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kWh, which is achievable given recent PPA tenders for wind and solar in many regions. The report by IRENA.ORG is an extensive factual report of present-day industrial hydrogen production consuming about 53 to 70 kWh per kg could go down to about 45 kWh/kg . The thermodynamic energy required for hydrogen by electrolysis translates to 33 kWh/kg, which is higher than steam reforming with carbon capture and higher than methane pyrolysis.
One of the advantages of electrolysis over hydrogen from steam methane reforming (SMR) is that the hydrogen can be produced on-site, meaning that the costly process of delivery via truck or pipeline is avoided.
Steam methane reforming is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis.
Chemically assisted electrolysis
In addition to reduce the voltage required for electrolysis via the increasing of the temperature of the electrolysis cell it is also possible to electrochemically consume the oxygen produced in an electrolyser by introducing a fuel (such as carbon/coal,methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
, ethanol, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
, glycerol, etc.) into the oxygen side of the reactor. This reduces the required electrical energy and has the potential to reduce the cost of hydrogen to less than 40~60% with the remaining energy provided in this manner.
Carbon/hydrocarbon assisted water electrolysis (CAWE) has the potential to offer a less energy intensive, cleaner method of using chemical energy in various sources of carbon, such as low-rank and high sulfur coals, biomass, alcohols and methane (Natural Gas), where pure CO2 produced can be easily sequestered without the need for separation.
Radiolysis
Nuclear radiation can break water bonds throughradiolysis
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is ...
. In the Mponeng
Mponeng is a gold mine in South Africa's Gauteng province. Previously known as Western Deep Levels #1 Shaft, the underground and surface works were commissioned in 1987. It extends over below the surface,. and is considered to be one of the most ...
gold mine, South Africa, researchers found bacteria in a naturally occurring high radiation zone. The bacterial community which was dominated by a new phylotype of ''Desulfotomaculum
''Desulfotomaculum'' is a genus of Gram-positive, obligately anaerobic soil bacteria. A type of sulfate-reducing bacteria, ''Desulfotomaculum'' can cause food spoilage in poorly processed canned foods. Their presence can be identified by the re ...
'', was feeding on primarily radiolytically produced hydrogen.
Thermolysis
Water spontaneously dissociates at around 2500 °C, but this thermolysis occurs at temperatures too high for usual process piping and equipment resulting in a rather low commercialization potential.Thermolysis via solar energy
Hydrogen production via water thermolysis based on solar energy involves using solar concentrators to directly collect solar energy to heat water to 2500 K, at which temperature it decomposes into H2 and O2. The heating temperature can be reduced by applying catalysts which allow the water decomposition by steps with lower energy.Pyrolysis on biomass
Pyrolysis can be divided into different types based on the pyrolysis temperature, namely low-temperature slow pyrolysis, medium-temperature rapid pyrolysis, and high-temperature flash pyrolysis. The source energy is mainly solar energy, with help of photosynthetic microorganisms to decompose water or biomass to produce hydrogen. However, this process has relatively low hydrogen yields and high operating cost. It is not a feasible method for industry.Nuclear-assisted thermolysis
The high-temperature gas-cooled reactor (HTGR) is one of the most promising CO2-free nuclear technique to produce hydrogen by splitting water in a large scale. In this method, iodine-sulfur (IS) thermo-chemical cycle for splitting water and high-temperature steam electrolysis (HTSE) were selected as the main processes for nuclear hydrogen production. The S-I cycle follows three chemical reactions: Bunsen reaction: I2+SO2+2H2O=H2SO4+2HI HI decomposition: 2HI=H2+I2 Sulfuric acid decomposition: H2SO4=SO2+1/2O2+H2O The hydrogen production rate of HTGR with IS cycle is approximately 0.68 kg/s, and the capital cost to build a unit of power plant is $100 million.Thermochemical cycle
Thermochemical cycles combine solely heat sources (''thermo'') with ''chemical'' reactions to split water into its hydrogen and oxygen components. The term ''cycle'' is used because aside from water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled. If electricity is partially used as an input, the resulting thermochemical cycle is defined as a hybrid one. The sulfur-iodine cycle (S-I cycle) is a thermochemical cycle processes which generates hydrogen from water with an efficiency of approximately 50%. The sulfur and iodine used in the process are recovered and reused, and not consumed by the process. The cycle can be performed with any source of very high temperatures, approximately 950 °C, such as by Concentrating solar power systems (CSP) and is regarded as being well suited to the production of hydrogen by high-temperature nuclear reactors, and as such, is being studied in the High-temperature engineering test reactor in Japan. There are other hybrid cycles that use both high temperatures and some electricity, such as the Copper–chlorine cycle, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction in one of the reaction steps, it operates at 530 °C and has an efficiency of 43 percent.Ferrosilicon method
Ferrosilicon is used by the military to quickly produce hydrogen forballoons
A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the per ...
. The chemical reaction uses sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
, ferrosilicon, and water. The generator is small enough to fit a truck and requires only a small amount of electric power, the materials are stable and not combustible, and they do not generate hydrogen until mixed. The method has been in use since World War I. A heavy steel pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
is filled with sodium hydroxide and ferrosilicon, closed, and a controlled amount of water is added; the dissolving of the hydroxide heats the mixture to about 93 °C and starts the reaction; sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate , sodium orthosilicate , and sodium pyrosilicate . The anions are often polymeric. These compounds are generally colorless transparent ...
, hydrogen and steam are produced.
Photobiological water splitting
 Biological hydrogen can be produced in an
Biological hydrogen can be produced in an algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
bioreactor. In the late 1990s it was discovered that if the algae are deprived of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
it will switch from the production of oxygen, i.e. normal photosynthesis, to the production of hydrogen. It seems that the production is now economically feasible by surpassing the 7–10 percent energy efficiency (the conversion of sunlight into hydrogen) barrier. with a hydrogen production rate of 10–12 ml per liter culture per hour.
Photocatalytic water splitting
The conversion of solar energy to hydrogen by means of water splitting process is one of the most interesting ways to achieve clean andrenewable energy
Renewable energy is energy that is collected from renewable resources that are naturally replenished on a human timescale. It includes sources such as sunlight, wind, the movement of water, and geothermal heat. Although most renewable energy ...
systems. However, if this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system the reaction is in just one step, it can be made more efficient.
Biohydrogen routes
Biomass
Biomass is plant-based material used as a fuel for heat or electricity production. It can be in the form of wood, wood residues, energy crops, agricultural residues, and waste from industry, farms, and households. Some people use the terms bi ...
and waste streams can in principle be converted into biohydrogen with biomass gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
, steam reforming, or biological conversion like biocatalysed electrolysis or fermentative hydrogen production.
Among hydrogen production methods such as steam methane reforming, thermal cracking, coal and biomass gasification and pyrolysis, electrolysis, and photolysis, biological ones are more eco-friendly and less energy intensive. In addition, a wide variety of waste and low-value materials such as agricultural biomass as renewable sources can be utilized to produce hydrogen via biochemical pathways. Nevertheless, at present hydrogen is produced mainly from fossil fuels, in particular, natural gas which are non-renewable sources. Hydrogen is not only the cleanest fuel but also widely used in a number of industries, especially fertilizer, petrochemical and food ones.
This makes it logical to investigate alternative sources for hydrogen production. The main biochemical technologies to produce hydrogen are dark and photo fermentation processes. In dark fermentation, carbohydrates are converted to hydrogen by fermentative microorganisms including strict anaerobe and facultative anaerobe bacteria. A theoretical maximum of 4 mol H2/mol glucose can be produced and, besides hydrogen, sugars are converted to volatile fatty acids (VFAs) and alcohols as by-products during this process. Photo fermentative bacteria are able to generate hydrogen from VFAs. Hence, metabolites formed in dark fermentation can be used as feedstock in photo fermentation to enhance the overall yield of hydrogen.
Fermentative hydrogen production
Biohydrogen can be produced in bioreactors. The process involves bacteria consuming hydrocarbons and producing hydrogen and CO2. The CO2 and hydrogen can be separated.Fermentative hydrogen production Fermentative hydrogen production is the fermentative conversion of organic substrates to H2. Hydrogen produced in this manner is often called biohydrogen. The conversion is effected by bacteria and protozoa, which employ enzymes. Fermentative h ...
is the fermentative conversion of organic substrate to biohydrogen manifested by a diverse group of bacteria using multi enzyme systems involving three steps similar to anaerobic conversion. Dark fermentation
Dark fermentation is the fermentative conversion of organic substrate to biohydrogen. It is a complex process manifested by diverse groups of bacteria, involving a series of biochemical reactions using three steps similar to anaerobic conversion ...
reactions do not require light energy, so they are capable of constantly producing hydrogen from organic compounds throughout the day and night. Photofermentation Photofermentation is the fermentative conversion of organic substrate to biohydrogen manifested by a diverse group of photosynthetic bacteria by a series of biochemical reactions involving three steps similar to anaerobic conversion. Photofermen ...
differs from dark fermentation
Dark fermentation is the fermentative conversion of organic substrate to biohydrogen. It is a complex process manifested by diverse groups of bacteria, involving a series of biochemical reactions using three steps similar to anaerobic conversion ...
because it only proceeds in the presence of light. For example, photo-fermentation with Rhodobacter sphaeroides SH2C can be employed to convert small molecular fatty acids into hydrogen.
Fermentative hydrogen production can be done using direct biophotolysis by green algae, indirect biophotolysis by cyanobacteria, photo-fermentation by anaerobic photosynthetic bacteria and dark fermentation by anaerobic fermentative bacteria. For example, studies on hydrogen production using ''H. salinarium'', an anaerobic photosynthetic bacteria, coupled to a hydrogenase donor like ''E. coli'', are reported in literature. ''Enterobacter aerogenes'' is another hydrogen producer.
Enzymatic hydrogen generation
Diverse enzymatic pathways have been designed to generate hydrogen from sugars.Biocatalysed electrolysis
 Besides dark fermentation, electrohydrogenesis (electrolysis using microbes) is another possibility. Using microbial fuel cells, wastewater or plants can be used to generate power. Biocatalysed electrolysis should not be confused with biological hydrogen production, as the latter only uses algae and with the latter, the algae itself generates the hydrogen instantly, where with biocatalysed electrolysis, this happens after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.
Besides dark fermentation, electrohydrogenesis (electrolysis using microbes) is another possibility. Using microbial fuel cells, wastewater or plants can be used to generate power. Biocatalysed electrolysis should not be confused with biological hydrogen production, as the latter only uses algae and with the latter, the algae itself generates the hydrogen instantly, where with biocatalysed electrolysis, this happens after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrass, cordgrass, rice, tomatoes, lupines and algae.

Nanogalvanic aluminum alloy powder
An aluminum alloy powder invented by theU.S. Army Research Laboratory
The U.S. Army Combat Capabilities Development Command Army Research Laboratory (DEVCOM ARL) is the U.S. Army's foundational research laboratory. ARL is headquartered at the Adelphi Laboratory Center (ALC) in Adelphi, Maryland. Its largest singl ...
in 2017 was shown to be capable of producing hydrogen gas upon contact with water or any liquid containing water due to its unique nanoscale galvanic microstructure. It reportedly generates hydrogen at 100 percent of the theoretical yield without the need for any catalysts, chemicals, or externally supplied power.
Environmental impact
As of 2020, most hydrogen is produced from fossil fuels, resulting in carbon dioxide emissions. This is often referred to as grey hydrogen when emissions are released to the atmosphere, and blue hydrogen when emissions are captured through carbon capture and storage (CCS). Blue hydrogen has been estimated to have agreenhouse gas footprint
A carbon footprint is the total greenhouse gas (GHG) emissions caused by an individual, event, organization, service, place or product, expressed as carbon dioxide equivalent (CO2e). Greenhouse gases, including the carbon-containing gases carbo ...
20% greater than burning gas or coal for heat and 60% greater when compared to burning diesel for heat, assuming US up- and mid-stream methane leakage rates and production via steam methane reformers (SMR) retrofitted with carbon dioxide capture.
The use of autothermal reformers (ATR) with integrated capture of carbon dioxide allow higher capture rates at satisfactory energy efficiencies and life cycle assessments have shown lower greenhouse gas emissions for such plants compared to SMRs with carbon dioxide capture. Application of ATR technology with integrated capture of carbon dioxide in Europe has been assessed to have a lower greenhouse gas footprint than burning natural gas, e.g. for the H21 project with a reported reduction of 68% due to a reduced carbon dioxide intensity of natural gas combined with a more suitable reactor type for capture of carbon dioxide.
Hydrogen produced using the newer, non-polluting technology methane pyrolysis is often referred to as turquoise hydrogen. High quality hydrogen is produced directly from natural gas and the associated non-polluting solid carbon is not released into the atmosphere and can then be sold for industrial use or stored in landfill.
Hydrogen produced from renewable energy
Renewable energy is energy that is collected from renewable resources that are naturally replenished on a human timescale. It includes sources such as sunlight, wind, the movement of water, and geothermal heat. Although most renewable energy ...
sources is often referred to as green hydrogen. There are two practical ways of producing hydrogen from renewable energy sources. One is to use power to gas, in which electric power is used to produce hydrogen from electrolysis of water, and the other is to use landfill gas to produce hydrogen in a steam reformer. Hydrogen fuel, when produced by renewable sources of energy like wind or solar power, is a renewable fuel. Hydrogen produced from nuclear energy
Nuclear energy may refer to:
*Nuclear power, the use of sustained nuclear fission or nuclear fusion to generate heat and electricity
* Nuclear binding energy, the energy needed to fuse or split a nucleus of an atom
*Nuclear potential energy
...
via electrolysis is sometimes viewed as a subset of green hydrogen, but can also be referred to as pink hydrogen. The Oskarshamn Nuclear Power Plant made an agreement in January 2022 to supply commercial pink hydrogen in the order of kilograms per day.
, estimated costs of production are $1–1.80/kg for grey hydrogen and blue hydrogen, and $2.50–6.80 for green hydrogen.
94 million tonnes of grey hydrogen are currently produced globally using fossil fuels as of 2022, primarily natural gas, and are therefore a significant source of greenhouse gas emissions.
Use of hydrogen
Hydrogen is used for the conversion of heavy petroleum fractions into lighter ones viahydrocracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of ...
. It is also used in other processes including the aromatization process, hydrodesulfurization
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of remov ...
and the production of ammonia via the Haber process, the primary industrial method for the production of synthetic nitrogen fertilizer for growing 47 percent of food worldwide.
Hydrogen may be used in fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
for local electricity generation or potentially as a transportation fuel.
Hydrogen is produced as a by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
of industrial chlorine production by electrolysis. Although requiring expensive technologies, hydrogen can be cooled, compressed and purified for use in other processes on site or sold to a customer via pipeline, cylinders or trucks. The discovery and development of less expensive methods of production of bulk hydrogen is relevant to the establishment of a hydrogen economy.
See also
*Ammonia production
Ammonia is one of the most highly produced inorganic chemicals. There are numerous large-scale ammonia plants worldwide, producing a grand total of 144 million tonnes of nitrogen (equivalent to 175 million tonnes of ammonia) in 2016. This has incr ...
* Artificial photosynthesis
* Biohydrogen
* Hydrogen analyzer
A hydrogen analyzer is a device used to measure the hydrogen concentration in steels and alloys. It also has industrial applications for corrosion monitoring.
See also
* Hydrogen embrittlement
* Hydrogen leak testing
Hydrogen leak testing is t ...
* Hydrogen compressor
*
* Hydrogen embrittlement
* Hydrogen leak testing
* Hydrogen pipeline transport
* Hydrogen purifier A hydrogen purifier is a device to purify hydrogen if hydrogen production is done from hydrocarbon sources, the ultra-high purified hydrogen is needed for applications like PEM fuel cells .
Palladium membrane hydrogen purifiers
The palladium memb ...
* Hydrogen purity
* Hydrogen safety
* Hydrogen sensor
A hydrogen sensor is a gas detector that detects the presence of hydrogen. They contain micro-fabricated point-contact hydrogen sensors and are used to locate hydrogen leaks. They are considered low-cost, compact, durable, and easy to maintain as ...
* Hydrogen storage
* Hydrogen station
* Hydrogen tank
* Hydrogen tanker
A hydrogen tanker is a tank ship designed for transporting liquefied hydrogen.
Research
The World Energy Network research program of the Japanese New Sunshine Project was divided into 3 phases during the period 1993 to 2002, its goal was to stud ...
* Hydrogen technologies
* Hydrogen valve
A hydrogen valve is a special type of valve that is used for hydrogen at very low temperatures or high pressures in hydrogen storage or for example hydrogen vehicles.
Types
High pressure ball valves up to 6000 psig (413 Bar (unit), bar) at 250 d ...
* Industrial gas
Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also avail ...
* Liquid hydrogen
* Next Generation Nuclear Plant
A Next Generation Nuclear Plant (NGNP) is a specific proposed generation IV reactor, generation IV very-high-temperature reactor (VHTR) that could be coupled to a neighboring hydrogen production facility. It could also produce electricity and supp ...
(partly for hydrogen production)
* Hy4Heat
* Lane hydrogen producer
The Lane hydrogen producer was an apparatus for hydrogen production based on the steam-iron process and water gas invented in 1903 by British engineer, Howard Lane.
History
The first commercial Lane hydrogen producer was commissioned in 1904. By ...
* Linde–Frank–Caro process The Linde–Frank–Caro process is a method for hydrogen production by removing hydrogen and carbon dioxide from water gas by condensation. The process was invented in 1909 by Adolf Frank and developed with Carl von Linde and Heinrich Caro. ...
* Underground hydrogen storage
Underground hydrogen storage is the practice of hydrogen storage in caverns, salt domes and depleted Oil field, oil/gas fields. Large quantities of gaseous hydrogen have been stored in caverns for many years. The storage of large quantities of hy ...
References
Further reading
* {{DEFAULTSORT:Hydrogen Production