Plutonium And Uranium Extraction From Nuclear Fuel-eng on:
[Wikipedia]
[Google]
[Amazon]
Plutonium is a
 Plutonium normally has six allotropes and forms a seventh (zeta, ζ) at high temperature within a limited pressure range. These allotropes, which are different structural modifications or forms of an element, have very similar internal energies but significantly varying
Plutonium normally has six allotropes and forms a seventh (zeta, ζ) at high temperature within a limited pressure range. These allotropes, which are different structural modifications or forms of an element, have very similar internal energies but significantly varying
 Plutonium is a radioactive
Plutonium is a radioactive
 Twenty
Twenty
+ -> -> beta^- 3.5 \ \ce -> beta^- .3565 \ \ce d
Neutrons from the fission of uranium-235 are captured by uranium-238 nuclei to form uranium-239; a
 At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized. The element displays four common ionic
At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized. The element displays four common ionic  Plutonium is a reactive metal. In moist air or moist
Plutonium is a reactive metal. In moist air or moist
 Plutonium (specifically, plutonium-238) was first produced, isolated and then chemically identified between December 1940 and February 1941 by
Plutonium (specifically, plutonium-238) was first produced, isolated and then chemically identified between December 1940 and February 1941 by
 The chemistry of plutonium was found to resemble uranium after a few months of initial study. Early research was continued at the secret Metallurgical Laboratory of the
The chemistry of plutonium was found to resemble uranium after a few months of initial study. Early research was continued at the secret Metallurgical Laboratory of the
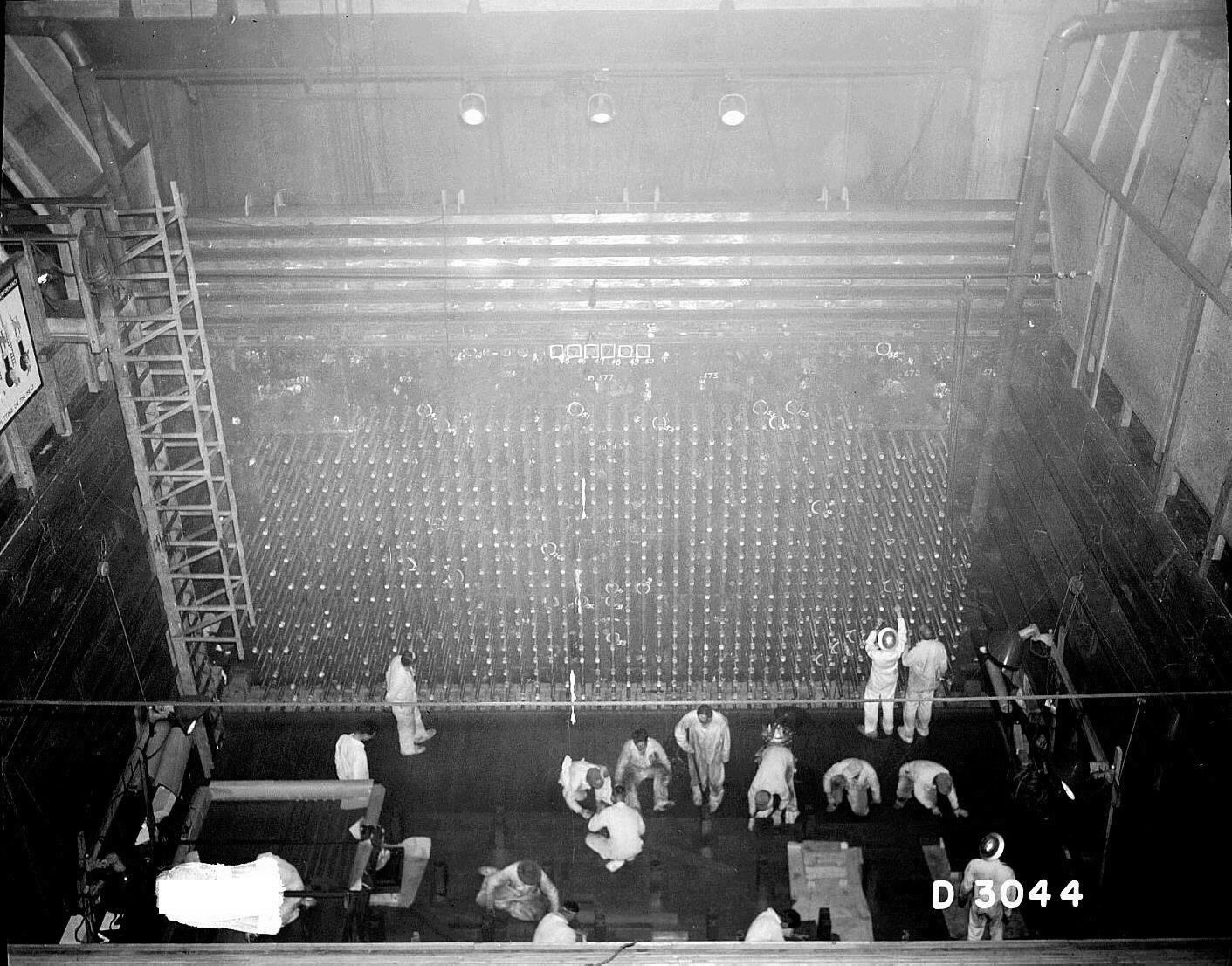 The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the
The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the
 The first atomic bomb test, codenamed "Trinity" and detonated on July 16, 1945, near Alamogordo, New Mexico, used plutonium as its fissile material. The implosion design of "
The first atomic bomb test, codenamed "Trinity" and detonated on July 16, 1945, near Alamogordo, New Mexico, used plutonium as its fissile material. The implosion design of "
 The isotope plutonium-239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's
The isotope plutonium-239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's

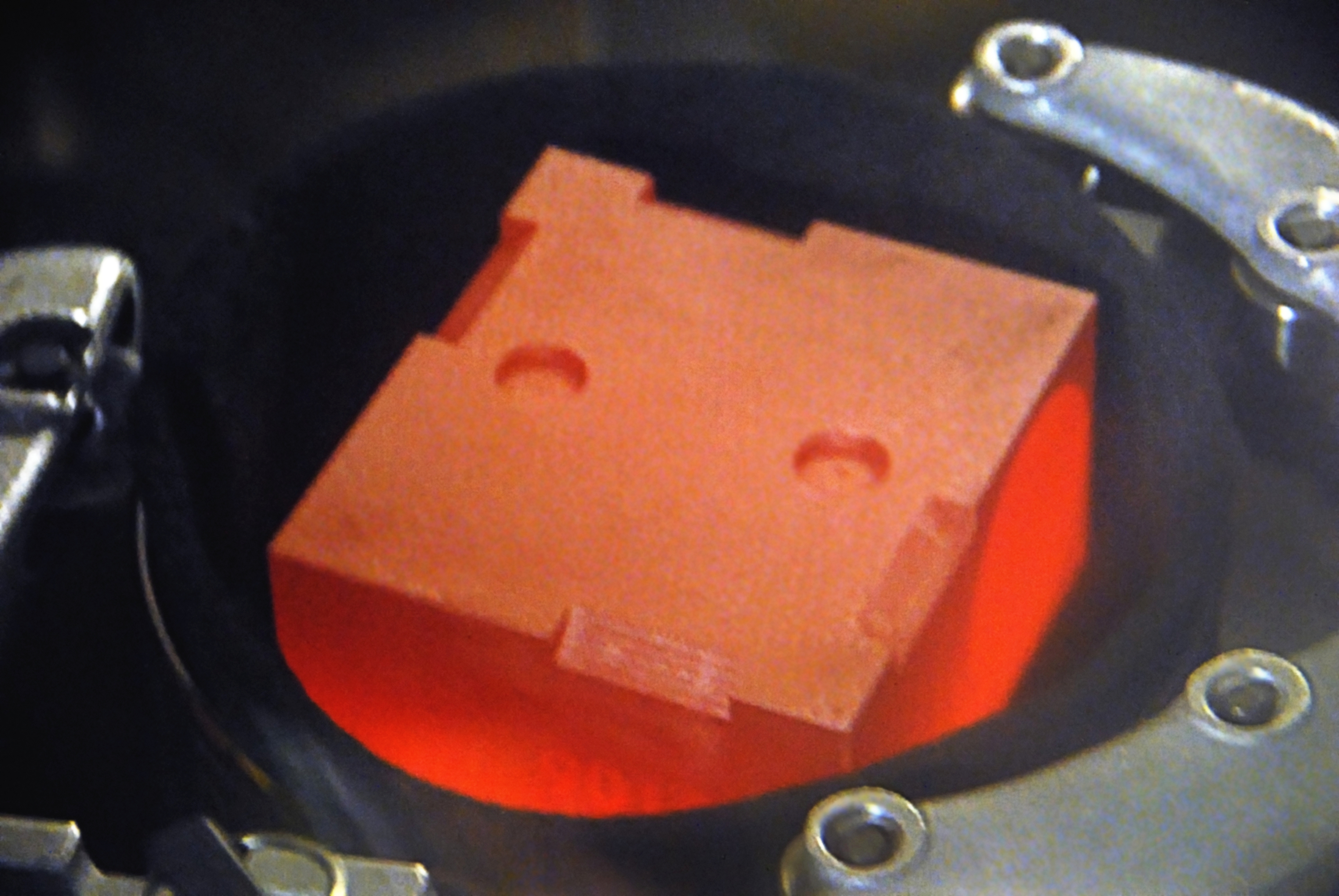 The isotope plutonium-238 has a half-life of 87.74 years. It emits a large amount of
The isotope plutonium-238 has a half-life of 87.74 years. It emits a large amount of
 Care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235. A critical mass of plutonium emits lethal amounts of neutrons and
Care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235. A critical mass of plutonium emits lethal amounts of neutrons and
radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Pu and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
94. It is an actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
of silvery-gray appearance that tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
es when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s. It reacts with carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, halogens, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. When exposed to moist air, it forms oxides and hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s that can expand the sample up to 70% in volume, which in turn flake off as a powder that is pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolit ...
. It is radioactive and can accumulate in bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, ...
s, which makes the handling of plutonium dangerous.
Plutonium was first synthetically produced and isolated in late 1940 and early 1941, by a deuteron
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
bombardment of uranium-238 in the cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
at the University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant u ...
. First, neptunium-238
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be sy ...
(half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
2.1 days) was synthesized, which subsequently beta-decayed to form the new element with atomic number 94 and atomic weight 238 (half-life 88 years). Since uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
had been named after the planet Uranus
Uranus is the seventh planet from the Sun. Its name is a reference to the Greek god of the sky, Uranus ( Caelus), who, according to Greek mythology, was the great-grandfather of Ares (Mars), grandfather of Zeus (Jupiter) and father of ...
and neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it bein ...
after the planet Neptune, element 94 was named after Pluto
Pluto (minor-planet designation: 134340 Pluto) is a dwarf planet in the Kuiper belt, a ring of bodies beyond the orbit of Neptune. It is the ninth-largest and tenth-most-massive known object to directly orbit the Sun. It is the largest ...
, which at the time was considered to be a planet as well. Wartime secrecy prevented the University of California team from publishing its discovery until 1948.
Plutonium is the element with the highest atomic number to occur in nature. Trace quantities arise in natural uranium-238 deposits when uranium-238 captures neutrons emitted by decay of other uranium-238 atoms.
Both plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
and plutonium-241
Plutonium-241 (241Pu or Pu-241) is an isotope of plutonium formed when plutonium-240 captures a neutron. Like some other plutonium isotopes (especially 239Pu), 241Pu is fissile, with a neutron absorption cross section about one-third greater t ...
are fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be t ...
, meaning that they can sustain a nuclear chain reaction, leading to applications in nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
s and nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s. Plutonium-240
Plutonium-240 ( or Pu-240) is an isotope of plutonium formed when plutonium-239 captures a neutron. The detection of its spontaneous fission led to its discovery in 1944 at Los Alamos and had important consequences for the Manhattan Project.
240 ...
exhibits a high rate of spontaneous fission, raising the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travellin ...
of any sample containing it. The presence of plutonium-240 limits a plutonium sample's usability for weapons or its quality as reactor fuel, and the percentage of plutonium-240 determines its grade
Grade most commonly refers to:
* Grade (education), a measurement of a student's performance
* Grade, the number of the year a student has reached in a given educational stage
* Grade (slope), the steepness of a slope
Grade or grading may also ref ...
(weapons-grade
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nucle ...
, fuel-grade, or reactor-grade). Plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suita ...
has a half-life of 87.7 years and emits alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
s. It is a heat source in radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s, which are used to power some spacecraft
A spacecraft is a vehicle or machine designed to fly in outer space. A type of artificial satellite, spacecraft are used for a variety of purposes, including communications, Earth observation, meteorology, navigation, space colonization, p ...
. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized reactors.
Producing plutonium in useful quantities for the first time was a major part of the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
during World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposing ...
that developed the first atomic bombs. The Fat Man
"Fat Man" (also known as Mark III) is the codename for the type of nuclear bomb the United States detonated over the Japanese city of Nagasaki on 9 August 1945. It was the second of the only two nuclear weapons ever used in warfare, the fir ...
bombs used in the Trinity
The Christian doctrine of the Trinity (, from 'threefold') is the central dogma concerning the nature of God in most Christian churches, which defines one God existing in three coequal, coeternal, consubstantial divine persons: God th ...
nuclear test
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, Nuclear weapon yield, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detona ...
in July 1945, and in the bombing of Nagasaki
The United States detonated two atomic bombs over the Japanese cities of Hiroshima and Nagasaki on 6 and 9 August 1945, respectively. The two bombings killed between 129,000 and 226,000 people, most of whom were civilians, and remain the on ...
in August 1945, had plutonium cores. Human radiation experiments
Since the discovery of ionizing radiation, a number of human radiation experiments have been performed to understand the effects of ionizing radiation and radioactive contamination on the human body, specifically with the element plutonium.
Ex ...
studying plutonium were conducted without informed consent, and several criticality accident
A criticality accident is an accidental uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, critical power excursion, or divergent chain reaction. Any such event involves the unintended accumulation ...
s, some lethal, occurred after the war. Disposal of plutonium waste from nuclear power plants and dismantled nuclear weapons built during the Cold War is a nuclear-proliferation and environmental concern. Other sources of plutonium in the environment are fallout
Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioac ...
from numerous above-ground nuclear tests, now banned.
Characteristics
Physical properties
Plutonium, like most metals, has a bright silvery appearance at first, much likenickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, but it oxidizes very quickly to a dull gray, although yellow and olive green are also reported. (public domain text) At room temperature plutonium is in its α (''alpha'') form. This, the most common structural form of the element ( allotrope), is about as hard and brittle as gray cast iron
Gray iron, or grey cast iron, is a type of cast iron that has a graphitic microstructure. It is named after the gray color of the fracture it forms, which is due to the presence of graphite.. It is the most common cast iron and the most widely u ...
unless it is alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
ed with other metals to make it soft and ductile. Unlike most metals, it is not a good conductor of heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ...
or electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as describ ...
. It has a low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
() and an unusually high boiling point (). This gives a large range of temperatures (over 2,500 kelvin wide) at which plutonium is liquid, but this range is neither the greatest among all actinides nor among all metals. The low melting point as well as the reactivity of the native metal compared to the oxide leads to plutonium oxides being a preferred form for applications such as nuclear fission reactor fuel ( MOX-fuel).
Alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
, the release of a high-energy helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
nucleus, is the most common form of radioactive decay for plutonium. A 5 kg mass of 239Pu contains about atoms. With a half-life of 24,100 years, about of its atoms decay each second by emitting a 5.157 MeV
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacu ...
alpha particle. This amounts to 9.68 watts of power. Heat produced by the deceleration of these alpha particles makes it warm to the touch. due to its much shorter half life heats up to much higher temperatures and glows red hot with blackbody radiation
Black-body radiation is the thermal electromagnetic radiation within, or surrounding, a body in thermodynamic equilibrium with its environment, emitted by a black body (an idealized opaque, non-reflective body). It has a specific, continuous spe ...
if left without external heating or cooling. This heat has been used in Radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s (see below).
Resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
is a measure of how strongly a material opposes the flow of electric current. The resistivity of plutonium at room temperature is very high for a metal, and it gets even higher with lower temperatures, which is unusual for metals. This trend continues down to 100 K, below which resistivity rapidly decreases for fresh samples. Resistivity then begins to increase with time at around 20 K due to radiation damage, with the rate dictated by the isotopic composition of the sample.
Because of self-irradiation, a sample of plutonium fatigues throughout its crystal structure, meaning the ordered arrangement of its atoms becomes disrupted by radiation with time. Self-irradiation can also lead to annealing which counteracts some of the fatigue effects as temperature increases above 100 K.
Unlike most materials, plutonium increases in density when it melts, by 2.5%, but the liquid metal exhibits a linear decrease in density with temperature. Near the melting point, the liquid plutonium has very high viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
and surface tension compared to other metals.
Allotropes
densities
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek language, Greek letter Rho (letter), rho), although the Latin letter ''D'' ca ...
and crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns ...
s. This makes plutonium very sensitive to changes in temperature, pressure, or chemistry, and allows for dramatic volume changes following phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states o ...
s from one allotropic form to another. The densities of the different allotropes vary from 16.00 g/cm3 to 19.86 g/cm3.
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. For example, the α form exists at room temperature in unalloyed plutonium. It has machining characteristics similar to cast iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impur ...
but changes to the plastic and malleable β (''beta'') form at slightly higher temperatures. The reasons for the complicated phase diagram are not entirely understood. The α form has a low-symmetry monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
structure, hence its brittleness, strength, compressibility, and poor thermal conductivity.
Plutonium in the δ (''delta'') form normally exists in the 310 °C to 452 °C range but is stable at room temperature when alloyed with a small percentage of gallium, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, or cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
, enhancing workability and allowing it to be welded
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as braz ...
. The δ form has more typical metallic character, and is roughly as strong and malleable as aluminium. In fission weapons, the explosive shock wave
In physics, a shock wave (also spelled shockwave), or shock, is a type of propagating disturbance that moves faster than the local speed of sound in the medium. Like an ordinary wave, a shock wave carries energy and can propagate through a me ...
s used to compress a plutonium core will also cause a transition from the usual δ phase plutonium to the denser α form, significantly helping to achieve supercriticality. The ε phase, the highest temperature solid allotrope, exhibits anomalously high atomic self-diffusion compared to other elements.
Nuclear fission
 Plutonium is a radioactive
Plutonium is a radioactive actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
metal whose isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
, plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
, is one of the three primary fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be t ...
isotopes (uranium-233
Uranium-233 (233U or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in exp ...
and uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
are the other two); plutonium-241
Plutonium-241 (241Pu or Pu-241) is an isotope of plutonium formed when plutonium-240 captures a neutron. Like some other plutonium isotopes (especially 239Pu), 241Pu is fissile, with a neutron absorption cross section about one-third greater t ...
is also highly fissile. To be considered fissile, an isotope's atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
must be able to break apart or fission when struck by a slow moving neutron and to release enough additional neutrons to sustain the nuclear chain reaction by splitting further nuclei.
Pure plutonium-239 may have a multiplication factor (keff) larger than one, which means that if the metal is present in sufficient quantity and with an appropriate geometry (e.g., a sphere of sufficient size), it can form a critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
. During fission, a fraction of the nuclear binding energy, which holds a nucleus together, is released as a large amount of electromagnetic and kinetic energy (much of the latter being quickly converted to thermal energy). Fission of a kilogram of plutonium-239 can produce an explosion equivalent to . It is this energy that makes plutonium-239 useful in nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
s and reactors.
The presence of the isotope plutonium-240
Plutonium-240 ( or Pu-240) is an isotope of plutonium formed when plutonium-239 captures a neutron. The detection of its spontaneous fission led to its discovery in 1944 at Los Alamos and had important consequences for the Manhattan Project.
240 ...
in a sample limits its nuclear bomb potential, as plutonium-240 has a relatively high spontaneous fission rate (~440 fissions per second per gram—over 1,000 neutrons per second per gram), raising the background neutron levels and thus increasing the risk of predetonation. Plutonium is identified as either weapons-grade
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nucle ...
, fuel-grade, or reactor-grade based on the percentage of plutonium-240 that it contains. Weapons-grade plutonium contains less than 7% plutonium-240. Fuel-grade plutonium contains from 7% to less than 19%, and power reactor-grade contains 19% or more plutonium-240. Supergrade plutonium
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main ...
, with less than 4% of plutonium-240, is used in U.S. Navy
The United States Navy (USN) is the maritime service branch of the United States Armed Forces and one of the eight uniformed services of the United States. It is the largest and most powerful navy in the world, with the estimated tonnage o ...
weapons stored in proximity to ship and submarine crews, due to its lower radioactivity. The isotope plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suita ...
is not fissile but can undergo nuclear fission easily with fast neutrons
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
as well as alpha decay. All plutonium isotopes can be "bred" into fissile material with one or more neutron absorption
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
s, whether followed by beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
or not. This makes non-fissile isotopes of Plutonium a fertile material
Fertile material is a material that, although not itself fissionable by thermal neutrons, can be converted into a fissile material by neutron absorption and subsequent nuclei conversions.
Naturally occurring fertile materials
Naturally occurring ...
.
Isotopes and nucleosynthesis
radioactive isotopes
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
of plutonium have been characterized. The longest-lived are plutonium-244, with a half-life of 80.8 million years, plutonium-242, with a half-life of 373,300 years, and plutonium-239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight metastable states, though all have half-lives less than one second. Plutonium-244 has been found in interstellar space and is has the longest half-life of any non-primordial radioisotope.
The known isotopes of plutonium range in mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
from 228 to 247. The primary decay modes of isotopes with mass numbers lower than the most stable isotope, plutonium-244, are spontaneous fission and alpha emission
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
, mostly forming uranium (92 protons) and neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it bein ...
(93 protons) isotopes as decay products (neglecting the wide range of daughter nuclei created by fission processes). The primary decay mode for isotopes with mass numbers higher than plutonium-244 is beta emission
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For exam ...
, mostly forming americium (95 protons) isotopes as decay products. Plutonium-241 is the parent isotope
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay direc ...
of the neptunium decay series, decaying to americium-241 via beta emission.
Plutonium-238 and 239 are the most widely synthesized isotopes. Plutonium-239 is synthesized via the following reaction using uranium (U) and neutrons (n) via beta decay (β−) with neptunium (Np) as an intermediate:
:beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
converts a neutron into a proton to form neptunium-239 (half-life 2.36 days) and another beta decay forms plutonium-239. Egon Bretscher
Egon Bretscher CBE (1901–1973) was a Swiss-born British chemist and nuclear physicist and Head of the Nuclear Physics Division from 1948 to 1966 at the Atomic Energy Research Establishment, also known as Harwell Laboratory, in Harwell, United ...
working on the British Tube Alloys
Tube Alloys was the research and development programme authorised by the United Kingdom, with participation from Canada, to develop nuclear weapons during the Second World War. Starting before the Manhattan Project in the United States, the ...
project predicted this reaction theoretically in 1940.
Plutonium-238 is synthesized by bombarding uranium-238 with deuteron
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
s (D, the nuclei of heavy hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
) in the following reaction:
:
In this process, a deuteron hitting uranium-238 produces two neutrons and neptunium-238, which spontaneously decays by emitting negative beta particles to form plutonium-238. Plutonium-238 can also be produced by neutron irradiation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emittin ...
of neptunium-237
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be sy ...
.
Decay heat and fission properties
Plutonium isotopes undergo radioactive decay, which producesdecay heat
Decay heat is the heat released as a result of radioactive decay. This heat is produced as an effect of radiation on materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.
Decay heat occur ...
. Different isotopes produce different amounts of heat per mass. The decay heat is usually listed as watt/kilogram, or milliwatt/gram. In larger pieces of plutonium (e.g. a weapon pit) and inadequate heat removal the resulting self-heating may be significant.
Compounds and chemistry
 At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized. The element displays four common ionic
At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized. The element displays four common ionic oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s in aqueous solution and one rare one:
* Pu(III), as Pu3+ (blue lavender)
* Pu(IV), as Pu4+ (yellow brown)
* Pu(V), as (light pink)
* Pu(VI), as (pink orange)
* Pu(VII), as (green)—the heptavalent ion is rare.
The color shown by plutonium solutions depends on both the oxidation state and the nature of the acid anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
. It is the acid anion that influences the degree of complexing—how atoms connect to a central atom—of the plutonium species. Additionally, the formal +2 oxidation state of plutonium is known in the complex (2.2.2-cryptand) uIICp″3 Cp″ = C5H3(SiMe3)2.
A +8 oxidation state is possible as well in the volatile tetroxide . Though it readily decomposes via a reduction mechanism similar to , can be stabilized in alkaline solutions and chloroform.
Metallic plutonium is produced by reacting plutonium tetrafluoride with barium, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
or lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
at 1200 °C. Metallic plutonium is attacked by acids, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, and steam but not by alkalis and dissolves easily in concentrated hydrochloric
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestiv ...
, hydroiodic and perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
s. Molten metal must be kept in a vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
or an inert atmosphere to avoid reaction with air. At 135 °C the metal will ignite in air and will explode if placed in carbon tetrachloride.
 Plutonium is a reactive metal. In moist air or moist
Plutonium is a reactive metal. In moist air or moist argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
, the metal oxidizes rapidly, producing a mixture of oxides and hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s. If the metal is exposed long enough to a limited amount of water vapor, a powdery surface coating of PuO2 is formed. Also formed is plutonium hydride
Plutonium hydride is a non-stoichiometric chemical compound with the formula PuH2+x. It is one of two characterised hydrides of plutonium, the other is PuH3.Gerd Meyer, 1991, Synthesis of Lanthanide and Actinide Compounds Springer, . PuH2 is non- ...
but an excess of water vapor forms only PuO2.
Plutonium shows enormous, and reversible, reaction rates with pure hydrogen, forming plutonium hydride
Plutonium hydride is a non-stoichiometric chemical compound with the formula PuH2+x. It is one of two characterised hydrides of plutonium, the other is PuH3.Gerd Meyer, 1991, Synthesis of Lanthanide and Actinide Compounds Springer, . PuH2 is non- ...
. It also reacts readily with oxygen, forming PuO and PuO2 as well as intermediate oxides; plutonium oxide fills 40% more volume than plutonium metal. The metal reacts with the halogens, giving rise to compounds with the general formula PuX3 where X can be F, Cl, Br or I and PuF4 is also seen. The following oxyhalides are observed: PuOCl, PuOBr and PuOI. It will react with carbon to form PuC, nitrogen to form PuN and silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
to form PuSi2.
The organometallic chemistry of plutonium complexes is typical for organoactinide
Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.
Like most organometallic compounds, the orga ...
species; a characteristic example of an organoplutonium compound is plutonocene
Plutonocene, Pu(C8H8)2, is an organoplutonium compound composed of a plutonium atom sandwiched between two cyclooctatetraenide (COT2-) rings. It is a dark red, very air-sensitive solid that is sparingly soluble in toluene and chlorocarbons. Plut ...
. Computational chemistry methods indicate an enhanced covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
character in the plutonium-ligand bonding.
Powders of plutonium, its hydrides and certain oxides like Pu2O3
are pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolit ...
, meaning they can ignite spontaneously at ambient temperature and are therefore handled in an inert, dry atmosphere of nitrogen or argon. Bulk plutonium ignites only when heated above 400 °C. Pu2O3 spontaneously heats up and transforms into PuO2, which is stable in dry air, but reacts with water vapor when heated.
Crucible
A crucible is a ceramic or metal container in which metals or other substances may be melted or subjected to very high temperatures. While crucibles were historically usually made from clay, they can be made from any material that withstands te ...
s used to contain plutonium need to be able to withstand its strongly reducing properties. Refractory metals
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definition of which elements belong to this group dif ...
such as tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that ...
and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
along with the more stable oxides, boride A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some bo ...
s, carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s, nitride
In chemistry, a nitride is an inorganic compound of nitrogen. The "nitride" anion, N3- ion, is very elusive but compounds of nitride are numerous, although rarely naturally occuring. Some nitrides have a find applications, such as wear-resistant ...
s and silicide
A silicide is a type of chemical compound that combines silicon and a (usually) more electropositive element.
Silicon is more electropositive than carbon. Silicides are structurally closer to borides than to carbides.
Similar to borides and carb ...
s can tolerate this. Melting in an electric arc furnace
An electric arc furnace (EAF) is a furnace that heats material by means of an electric arc.
Industrial arc furnaces range in size from small units of approximately one-tonne capacity (used in foundries for producing cast iron products) up to ...
can be used to produce small ingots of the metal without the need for a crucible.
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
is used as a chemical simulant of plutonium for development of containment, extraction, and other technologies.
Electronic structure
Plutonium is an element in which the 5f electrons are the transition border between delocalized and localized; it is therefore considered one of the most complex elements. The anomalous behavior of plutonium is caused by its electronic structure. The energy difference between the 6d and 5f subshells is very low. The size of the 5f shell is just enough to allow the electrons to form bonds within the lattice, on the very boundary between localized and bonding behavior. The proximity of energy levels leads to multiple low-energy electron configurations with near equal energy levels. This leads to competing 5fn7s2 and 5fn−16d17s2 configurations, which causes the complexity of its chemical behavior. The highly directional nature of 5f orbitals is responsible for directional covalent bonds in molecules and complexes of plutonium.Alloys
Plutonium can form alloys and intermediate compounds with most other metals. Exceptions include lithium,sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
, potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
, rubidium and caesium of the alkali metals; and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, calcium, strontium, and barium of the alkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
s; and europium and ytterbium
Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. However, like the othe ...
of the rare earth metals. Partial exceptions include the refractory metals chromium, molybdenum, niobium, tantalum, and tungsten, which are soluble in liquid plutonium, but insoluble or only slightly soluble in solid plutonium. Gallium, aluminium, americium, scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
and cerium can stabilize the δ phase of plutonium for room temperature. Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
and zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
allow formation of metastable δ state when rapidly cooled. High amounts of hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
, holmium
Holmium is a chemical element with the symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like a lot of oth ...
and thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
also allows some retention of the δ phase at room temperature. Neptunium is the only element that can stabilize the α phase at higher temperatures.
Plutonium alloys can be produced by adding a metal to molten plutonium. If the alloying metal is sufficiently reductive, plutonium can be added in the form of oxides or halides. The δ phase plutonium–gallium and plutonium–aluminium alloys are produced by adding plutonium(III) fluoride to molten gallium or aluminium, which has the advantage of avoiding dealing directly with the highly reactive plutonium metal.
* Plutonium–gallium is used for stabilizing the δ phase of plutonium, avoiding the α-phase and α–δ related issues. Its main use is in pits of implosion nuclear weapons.
* Plutonium–aluminium is an alternative to the Pu–Ga alloy. It was the original element considered for δ phase stabilization, but its tendency to react with the alpha particles and release neutrons reduces its usability for nuclear weapon pits. Plutonium–aluminium alloy can be also used as a component of nuclear fuel.
* Plutonium–gallium–cobalt alloy (PuCoGa5) is an unconventional superconductor
Unconventional superconductors are materials that display superconductivity which does not conform to either the conventional BCS theory or Nikolay Bogolyubov's theory or its extensions.
History
The superconducting properties of CeCu2Si2, a t ...
, showing superconductivity below 18.5 K, an order of magnitude higher than the highest between heavy fermion
In solid-state physics, heavy fermion materials are a specific type of intermetallic compound, containing elements with 4f or 5f electrons in unfilled electron bands. Electrons are one type of fermion, and when they are found in such materials, t ...
systems, and has large critical current.
* Plutonium–zirconium alloy can be used as nuclear fuel.
* Plutonium–cerium and plutonium–cerium–cobalt alloys are used as nuclear fuels.
* Plutonium–uranium, with about 15–30 mol.% plutonium, can be used as a nuclear fuel for fast breeder reactors. Its pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolit ...
nature and high susceptibility to corrosion to the point of self-igniting or disintegrating after exposure to air require alloying with other components. Addition of aluminium, carbon or copper does not improve disintegration rates markedly, zirconium and iron alloys have better corrosion resistance but they disintegrate in several months in air as well. Addition of titanium and/or zirconium significantly increases the melting point of the alloy.
* Plutonium–uranium–titanium and plutonium–uranium–zirconium were investigated for use as nuclear fuels. The addition of the third element increases corrosion resistance, reduces flammability, and improves ductility, fabricability, strength, and thermal expansion. Plutonium–uranium–molybdenum has the best corrosion resistance, forming a protective film of oxides, but titanium and zirconium are preferred for physics reasons.
* Thorium–uranium–plutonium was investigated as a nuclear fuel for fast breeder reactors.
Occurrence
Trace amounts of plutonium-238, plutonium-239, plutonium-240, and plutonium-244 can be found in nature. Small traces of plutonium-239, a fewparts per trillion
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they ...
, and its decay products are naturally found in some concentrated ores of uranium, such as the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
in Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a Fren ...
, Gabon
Gabon (; ; snq, Ngabu), officially the Gabonese Republic (french: République gabonaise), is a country on the west coast of Central Africa. Located on the equator, it is bordered by Equatorial Guinea to the northwest, Cameroon to the nort ...
. The ratio of plutonium-239 to uranium at the Cigar Lake Mine
The Cigar Lake Mine is a large high-grade underground uranium mine, located in the uranium-rich Athabasca Basin of northern Saskatchewan, Canada, at the south-west corner of Waterbury Lake. The deposit, discovered in 1981, is second in size of h ...
uranium deposit ranges from to . These trace amounts of 239Pu originate in the following fashion: on rare occasions, 238U undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy. When one of these neutrons strikes the nucleus of another 238U atom, it is absorbed by the atom, which becomes 239U. With a relatively short half-life, 239U decays to 239Np, which decays into 239Pu. Finally, exceedingly small amounts of plutonium-238, attributed to the extremely rare double beta decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move clos ...
of uranium-238, have been found in natural uranium samples.
Due to its relatively long half-life of about 80 million years, it was suggested that plutonium-244
Plutonium-244 (244Pu) is an isotope of plutonium that has a half-life of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any other actinide isotope except for the three naturally abundant ones: uranium ...
occurs naturally as a primordial nuclide, but early reports of its detection could not be confirmed. However, its long half-life ensured its circulation across the solar system before its extinction
Extinction is the termination of a kind of organism or of a group of kinds (taxon), usually a species. The moment of extinction is generally considered to be the death of the last individual of the species, although the capacity to breed and ...
, and indeed, evidence of the spontaneous fission of extinct 244Pu has been found in meteorites. The former presence of 244Pu in the early Solar System has been confirmed, since it manifests itself today as an excess of its daughters, either 232 Th (from the alpha decay pathway) or xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
isotopes (from its spontaneous fission). The latter are generally more useful, because the chemistries of thorium and plutonium are rather similar (both are predominantly tetravalent) and hence an excess of thorium would not be strong evidence that some of it was formed as a plutonium daughter. 244Pu has the longest half-life of all transuranic nuclides and is produced only in the r-process in supernovae and colliding neutron star
A neutron star is the collapsed core of a massive supergiant star, which had a total mass of between 10 and 25 solar masses, possibly more if the star was especially metal-rich. Except for black holes and some hypothetical objects (e.g. w ...
s; when nuclei are ejected from these events at high speed to reach Earth, 244Pu alone among transuranic nuclides has a long enough half-life to survive the journey, and hence tiny traces of live interstellar 244Pu have been found in the deep sea floor. Because 240Pu also occurs in the decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
of 244Pu, it must thus also be present in secular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate (e.g., due to decay of a parent isotope) is equal to its decay rate.
In radioactive decay
Secular e ...
, albeit in even tinier quantities.
Minute traces of plutonium are usually found in the human body due to the 550 atmospheric and underwater nuclear tests
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected by ...
that have been carried out, and to a small number of major nuclear accidents
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, lar ...
. Most atmospheric and underwater nuclear testing was stopped by the Limited Test Ban Treaty
The Partial Test Ban Treaty (PTBT) is the abbreviated name of the 1963 Treaty Banning Nuclear Weapon Tests in the Atmosphere, in Outer Space and Under Water, which prohibited all test detonations of nuclear weapons except for those conducted ...
in 1963, which of the nuclear powers was signed and ratified by the United States, United Kingdom and Soviet Union
The Soviet Union,. officially the Union of Soviet Socialist Republics. (USSR),. was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 to 1991. A flagship communist state, ...
. France would continue atmospheric nuclear testing until 1974 and China would continue atmospheric nuclear testing until 1980. All subsequent nuclear testing was conducted underground.
History
Discovery
Enrico Fermi and a team of scientists at the University of Rome reported that they had discovered element 94 in 1934. Fermi called the element ''hesperium
Hesperium (or esperium; atomic symbol Es) was the name assigned to the element with atomic number 94, now known as plutonium.
It was named in Italian ''Esperio'' after a Greek name of Italy, Hesperia, "the land of the West".
The same team assigned ...
'' and mentioned it in his Nobel Lecture in 1938. The sample actually contained products of nuclear fission, primarily barium and krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often ...
. Nuclear fission, discovered in Germany in 1938 by Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the fields of radioactivity and radiochemistry. He is referred to as the father of nuclear chemistry and father of nuclear fission. Hahn and Lise Meitner ...
and Fritz Strassmann
Friedrich Wilhelm Strassmann (; 22 February 1902 – 22 April 1980) was a German chemist who, with Otto Hahn in December 1938, identified the element barium as a product of the bombardment of uranium with neutrons. Their observation was the ke ...
, was unknown at the time.
 Plutonium (specifically, plutonium-238) was first produced, isolated and then chemically identified between December 1940 and February 1941 by
Plutonium (specifically, plutonium-238) was first produced, isolated and then chemically identified between December 1940 and February 1941 by Glenn T. Seaborg
Glenn Theodore Seaborg (; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work i ...
, Edwin McMillan
Edwin Mattison McMillan (September 18, 1907 – September 7, 1991) was an American physicist credited with being the first-ever to produce a transuranium element, neptunium. For this, he shared the 1951 Nobel Prize in Chemistry with Glenn Seab ...
, Emilio Segrè
Emilio Gino Segrè (1 February 1905 – 22 April 1989) was an Italian-American physicist and Nobel laureate, who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was awarded the Nobe ...
, Joseph W. Kennedy, and Arthur Wahl
Arthur Charles Wahl (September 8, 1917 – March 6, 2006) was an American chemist who, as a doctoral student of Glenn T. Seaborg at the University of California, Berkeley, first isolated plutonium in February 1941.cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
at the Berkeley Radiation Laboratory at the University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant u ...
.
Neptunium-238 was created directly by the bombardment but decayed by beta emission with a half-life of a little over two days, which indicated the formation of element 94. The first bombardment took place on December 14, 1940, and the new element was first identified through oxidation on the night of February 23–24, 1941.
A paper documenting the discovery was prepared by the team and sent to the journal '' Physical Review'' in March 1941, but publication was delayed until a year after the end of World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposing ...
due to security concerns. At the Cavendish Laboratory in Cambridge
Cambridge ( ) is a College town, university city and the county town in Cambridgeshire, England. It is located on the River Cam approximately north of London. As of the 2021 United Kingdom census, the population of Cambridge was 145,700. Cam ...
, Egon Bretscher and Norman Feather
Norman Feather FRS FRSE PRSE (16 November 1904 – 14 August 1978), was an English nuclear physicist. Feather and Egon Bretscher were working at the Cavendish Laboratory, Cambridge in 1940, when they proposed that the 239 isotope of element ...
realized that a slow neutron reactor fuelled with uranium would theoretically produce substantial amounts of plutonium-239 as a by-product. They calculated that element 94 would be fissile, and had the added advantage of being chemically different from uranium, and could easily be separated from it.
McMillan had recently named the first transuranic element neptunium after the planet Neptune, and suggested that element 94, being the next element in the series, be named for what was then considered the next planet, Pluto
Pluto (minor-planet designation: 134340 Pluto) is a dwarf planet in the Kuiper belt, a ring of bodies beyond the orbit of Neptune. It is the ninth-largest and tenth-most-massive known object to directly orbit the Sun. It is the largest ...
. Nicholas Kemmer
Nicholas Kemmer (7 December 1911 – 21 October 1998) was a Russian-born nuclear physicist working in Britain, who played an integral and leading edge role in United Kingdom's nuclear programme, and was known as a mentor of Abdus Salam – a N ...
of the Cambridge team independently proposed the same name, based on the same reasoning as the Berkeley team. Seaborg originally considered the name "plutium", but later thought that it did not sound as good as "plutonium". He chose the letters "Pu" as a joke, in reference to the interjection "P U" to indicate an especially disgusting smell, which passed without notice into the periodic table. Alternative names considered by Seaborg and others were "ultimium" or "extremium" because of the erroneous belief that they had found the last possible element on the periodic table.
Hahn and Strassmann, and independently Kurt Starke, were at this point also working on transuranic elements in Berlin. It is likely that Hahn and Strassmann were aware that plutonium-239 should be fissile. However, they did not have a strong neutron source. Element 93 was reported by Hahn and Strassmann, as well as Starke, in 1942. Hahn's group did not pursue element 94, likely because they were discouraged by McMillan and Abelson's lack of success in isolating it when they had first found element 93. However, since Hahn's group had access to the stronger cyclotron at Paris at this point, they would likely have been able to detect plutonium had they tried, albeit in tiny quantities (a few becquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. For applications relatin ...
s).
Early research
 The chemistry of plutonium was found to resemble uranium after a few months of initial study. Early research was continued at the secret Metallurgical Laboratory of the
The chemistry of plutonium was found to resemble uranium after a few months of initial study. Early research was continued at the secret Metallurgical Laboratory of the University of Chicago
The University of Chicago (UChicago, Chicago, U of C, or UChi) is a private university, private research university in Chicago, Illinois. Its main campus is located in Chicago's Hyde Park, Chicago, Hyde Park neighborhood. The University of Chic ...
. On August 20, 1942, a trace quantity of this element was isolated and measured for the first time. About 50 micrograms of plutonium-239 combined with uranium and fission products was produced and only about 1 microgram was isolated. This procedure enabled chemists to determine the new element's atomic weight.
On December 2, 1942, on a racket court under the west grandstand at the University of Chicago's Stagg Field, researchers headed by Enrico Fermi achieved the first self-sustaining chain reaction in a graphite and uranium pile known as CP-1. Using theoretical information garnered from the operation of CP-1, DuPont constructed an air-cooled experimental production reactor, known as X-10, and a pilot chemical separation facility at Oak Ridge. The separation facility, using methods developed by Glenn T. Seaborg and a team of researchers at the Met Lab, removed plutonium from uranium irradiated in the X-10 reactor. Information from CP-1 was also useful to Met Lab scientists designing the water-cooled plutonium production reactors for Hanford. Construction at the site began in mid-1943.
In November 1943 some plutonium trifluoride was reduced to create the first sample of plutonium metal: a few micrograms of metallic beads. Enough plutonium was produced to make it the first synthetically made element to be visible with the unaided eye.
The nuclear properties of plutonium-239 were also studied; researchers found that when it is hit by a neutron it breaks apart (fissions) by releasing more neutrons and energy. These neutrons can hit other atoms of plutonium-239 and so on in an exponentially fast chain reaction. This can result in an explosion large enough to destroy a city if enough of the isotope is concentrated to form a critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
.
During the early stages of research, animals were used to study the effects of radioactive substances on health. These studies began in 1944 at the University of California at Berkeley's Radiation Laboratory and were conducted by Joseph G. Hamilton. Hamilton was looking to answer questions about how plutonium would vary in the body depending on exposure mode (oral ingestion, inhalation, absorption through skin), retention rates, and how plutonium would be fixed in tissues and distributed among the various organs. Hamilton started administering soluble microgram portions of plutonium-239 compounds to rats using different valence states and different methods of introducing the plutonium (oral, intravenous, etc.). Eventually, the lab at Chicago also conducted its own plutonium injection experiments using different animals such as mice, rabbits, fish, and even dogs. The results of the studies at Berkeley and Chicago showed that plutonium's physiological behavior differed significantly from that of radium. The most alarming result was that there was significant deposition of plutonium in the liver and in the "actively metabolizing" portion of bone. Furthermore, the rate of plutonium elimination in the excreta differed between species of animals by as much as a factor of five. Such variation made it extremely difficult to estimate what the rate would be for human beings.
Production during the Manhattan Project
During World War II the U.S. government established theManhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, which was tasked with developing an atomic bomb. The three primary research and production sites of the project were the plutonium production facility at what is now the Hanford Site
The Hanford Site is a decommissioned nuclear production complex operated by the United States federal government on the Columbia River in Benton County in the U.S. state of Washington. The site has been known by many names, including SiteW a ...
, the uranium enrichment
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238 ...
facilities at Oak Ridge, Tennessee, and the weapons research and design laboratory, now known as Los Alamos National Laboratory
Los Alamos National Laboratory (often shortened as Los Alamos and LANL) is one of the sixteen research and development laboratories of the United States Department of Energy (DOE), located a short distance northwest of Santa Fe, New Mexico, ...
.
 The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the
The first production reactor that made plutonium-239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research an ...
.
In January 1944, workers laid the foundations for the first chemical separation building, T Plant located in 200-West. Both the T Plant and its sister facility in 200-West, the U Plant, were completed by October. (U Plant was used only for training during the Manhattan Project.) The separation building in 200-East, B Plant, was completed in February 1945. The second facility planned for 200-East was canceled. Nicknamed Queen Marys by the workers who built them, the separation buildings were awesome canyon-like structures 800 feet long, 65 feet wide, and 80 feet high containing forty process pools. The interior had an eerie quality as operators behind seven feet of concrete shielding manipulated remote control equipment by looking through television monitors and periscopes from an upper gallery. Even with massive concrete lids on the process pools, precautions against radiation exposure were necessary and influenced all aspects of plant design.
On April 5, 1944, Emilio Segrè
Emilio Gino Segrè (1 February 1905 – 22 April 1989) was an Italian-American physicist and Nobel laureate, who discovered the elements technetium and astatine, and the antiproton, a subatomic antiparticle, for which he was awarded the Nobe ...
at Los Alamos received the first sample of reactor-produced plutonium from Oak Ridge. Within ten days, he discovered that reactor-bred plutonium had a higher concentration of the isotope plutonium-240 than cyclotron-produced plutonium. Plutonium-240 has a high spontaneous fission rate, raising the overall background neutron level of the plutonium sample. The original gun-type plutonium weapon, code-named " Thin Man", had to be abandoned as a result—the increased number of spontaneous neutrons meant that nuclear pre-detonation ( fizzle) was likely.
The entire plutonium weapon design effort at Los Alamos was soon changed to the more complicated implosion device, code-named "Fat Man
"Fat Man" (also known as Mark III) is the codename for the type of nuclear bomb the United States detonated over the Japanese city of Nagasaki on 9 August 1945. It was the second of the only two nuclear weapons ever used in warfare, the fir ...
". With an implosion weapon, plutonium is compressed to a high density with explosive lens
An explosive lens—as used, for example, in nuclear weapons—is a highly specialized shaped charge. In general, it is a device composed of several explosive charges. These charges are arranged and formed with the intent to control the shape ...
es—a technically more daunting task than the simple gun-type design, but necessary to use plutonium for weapons purposes. Enriched uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U ...
, by contrast, can be used with either method.
Construction of the Hanford B Reactor
The B Reactor at the Hanford Site, near Richland, Washington, was the first large-scale nuclear reactor ever built. The project was a key part of the Manhattan Project, the United States nuclear weapons development program during World War II. I ...
, the first industrial-sized nuclear reactor for the purposes of material production, was completed in March 1945. B Reactor produced the fissile material for the plutonium weapons used during World War II. B, D and F were the initial reactors built at Hanford, and six additional plutonium-producing reactors were built later at the site.
By the end of January 1945, the highly purified plutonium underwent further concentration in the completed chemical isolation building, where remaining impurities were removed successfully. Los Alamos received its first plutonium from Hanford on February 2. While it was still by no means clear that enough plutonium could be produced for use in bombs by the war's end, Hanford was by early 1945 in operation. Only two years had passed since Col. Franklin Matthias
Franklin Thompson Matthias (13 March 1908 – 3 December 1993) was an American civil engineer who directed the construction of the Hanford nuclear site, a key facility of the Manhattan Project during World War II.
A graduate of the University ...
first set up his temporary headquarters on the banks of the Columbia River.
According to Kate Brown
Katherine Brown (born June 21, 1960) is an American politician and attorney serving as the 38th governor of Oregon since 2015. A member of the Democratic Party, she served three terms as the state representative from the 13th district of the ...
, the plutonium production plants at Hanford and Mayak
The Mayak Production Association (russian: Производственное объединение «Маяк», , from 'lighthouse') is one of the biggest nuclear facilities in the Russian Federation, housing a reprocessing plant. The closest ...
in Russia, over a period of four decades, "both released more than 200 million curies of radioactive isotopes into the surrounding environment—twice the amount expelled in the Chernobyl disaster in each instance". Most of this radioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is unintended or undesirab ...
over the years were part of normal operations, but unforeseen accidents did occur and plant management kept this secret, as the pollution continued unabated.
In 2004, a safe was discovered during excavations of a burial trench at the Hanford nuclear site. Inside the safe were various items, including a large glass bottle containing a whitish slurry which was subsequently identified as the oldest sample of weapons-grade plutonium known to exist. Isotope analysis by Pacific Northwest National Laboratory indicated that the plutonium in the bottle was manufactured in the X-10 Graphite Reactor at Oak Ridge during 1944.
Trinity and Fat Man atomic bombs
the gadget
Trinity was the code name of the first detonation of a nuclear weapon. It was conducted by the United States Army at 5:29 a.m. on July 16, 1945, as part of the Manhattan Project. The test was conducted in the Jornada del Muerto desert abo ...
", as the Trinity device was code-named, used conventional explosive lenses to compress a sphere of plutonium into a supercritical mass, which was simultaneously showered with neutrons from the "Urchin", an initiator made of polonium
Polonium is a chemical element with the symbol Po and atomic number 84. Polonium is a chalcogen. A rare and highly radioactive metal with no stable isotopes, polonium is chemically similar to selenium and tellurium, though its metallic character ...
and beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form m ...
(neutron source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear p ...
: (α, n) reaction). Together, these ensured a runaway chain reaction and explosion. The overall weapon weighed over 4 tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
s, although it used just 6.2 kg of plutonium in its core. About 20% of the plutonium used in the Trinity weapon underwent fission, resulting in an explosion with an energy equivalent to approximately 20,000 tons of TNT.
An identical design was used in the "Fat Man" atomic bomb dropped on Nagasaki
is the capital and the largest Cities of Japan, city of Nagasaki Prefecture on the island of Kyushu in Japan.
It became the sole Nanban trade, port used for trade with the Portuguese and Dutch during the 16th through 19th centuries. The Hi ...
, Japan, on August 9, 1945, killing 35,000–40,000 people and destroying 68%–80% of war production at Nagasaki. Only after the announcement of the first atomic bombs was the existence and name of plutonium made known to the public by the Manhattan Project's Smyth Report
The Smyth Report (officially ''Atomic Energy for Military Purposes'') is the common name of an administrative history written by American physicist Henry DeWolf Smyth about the Manhattan Project, the Allied effort to develop atomic bombs du ...
.
Cold War use and waste
Large stockpiles ofweapons-grade plutonium
Weapons-grade nuclear material is any fissionable nuclear material that is pure enough to make a nuclear weapon or has properties that make it particularly suitable for nuclear weapons use. Plutonium and uranium in grades normally used in nucle ...
were built up by both the Soviet Union
The Soviet Union,. officially the Union of Soviet Socialist Republics. (USSR),. was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 to 1991. A flagship communist state, ...
and the United States during the Cold War. The U.S. reactors at Hanford and the Savannah River Site
The Savannah River Site (SRS) is a U.S. Department of Energy (DOE) reservation in the United States in the state of South Carolina, located on land in Aiken, Allendale, and Barnwell counties adjacent to the Savannah River, southeast of August ...
in South Carolina produced 103 tonnes, and an estimated 170 tonnes of military-grade plutonium was produced in the USSR. Each year about 20 tonnes of the element is still produced as a by-product of the nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced ...
industry. As much as 1000 tonnes of plutonium may be in storage with more than 200 tonnes of that either inside or extracted from nuclear weapons.
SIPRI Sipri may refer to:
* As-Safira, a city in Syria, known in pre-Islamic times as Sipri
*Shivpuri, a city and a municipality in Madhya Pradesh, India, formerly known as Sipri
*Stockholm International Peace Research Institute
Stockholm Internati ...
estimated the world plutonium stockpile in 2007 as about 500 tonnes, divided equally between weapon and civilian stocks.
Radioactive contamination at the Rocky Flats Plant
The Rocky Flats Plant was a U.S. manufacturing complex that produced nuclear weapons parts in the western United States, near Denver, Colorado. The facility's primary mission was the fabrication of plutonium pits, which were shipped to ot ...
primarily resulted from two major plutonium fires in 1957 and 1969. Much lower concentrations of radioactive isotopes were released throughout the operational life of the plant from 1952 to 1992. Prevailing winds from the plant carried airborne contamination south and east, into populated areas northwest of Denver. The contamination of the Denver area by plutonium from the fires and other sources was not publicly reported until the 1970s. According to a 1972 study coauthored by Edward Martell, "In the more densely populated areas of Denver, the Pu contamination level in surface soils is several times fallout", and the plutonium contamination "just east of the Rocky Flats plant ranges up to hundreds of times that from nuclear tests". As noted by Carl Johnson in Ambio
''Ambio: A Journal of Environment and Society'' is a monthly peer-reviewed scientific journal published by Springer Science+Business Media on behalf of the Royal Swedish Academy of Sciences. It was established in 1972. The editor-in-chief is Bo ...
, "Exposures of a large population in the Denver area to plutonium and other radionuclides in the exhaust plumes from the plant date back to 1953." Reprinted in Weapons production at the Rocky Flats plant was halted after a combined FBI
The Federal Bureau of Investigation (FBI) is the domestic intelligence and security service of the United States and its principal federal law enforcement agency. Operating under the jurisdiction of the United States Department of Justice, t ...
and EPA
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
raid in 1989 and years of protests. The plant has since been shut down, with its buildings demolished and completely removed from the site.
In the U.S., some plutonium extracted from dismantled nuclear weapons is melted to form glass logs of plutonium oxide that weigh two tonnes. The glass is made of borosilicate
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion (≈3 × 10−6 K−1 at 20 °C), ma ...
s mixed with cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
and gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
. These logs are planned to be encased in stainless steel and stored as much as underground in bore holes that will be back-filled with concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most wid ...
. The U.S. planned to store plutonium in this way at the Yucca Mountain nuclear waste repository
The Yucca Mountain Nuclear Waste Repository, as designated by the Nuclear Waste Policy Act amendments of 1987, is a proposed deep geological repository storage facility within Yucca Mountain for spent nuclear fuel and other high-level radio ...
, which is about north-east of Las Vegas, Nevada
Las Vegas (; Spanish for "The Meadows"), often known simply as Vegas, is the 25th-most populous city in the United States, the most populous city in the state of Nevada, and the county seat of Clark County. The city anchors the Las Vega ...
.
On March 5, 2009, Energy Secretary Steven Chu told a Senate hearing "the Yucca Mountain site no longer was viewed as an option for storing reactor waste". Starting in 1999, military-generated nuclear waste is being entombed at the Waste Isolation Pilot Plant
The Waste Isolation Pilot Plant, or WIPP, is the world's third deep geological repository (after Germany's Repository for radioactive waste Morsleben and the Schacht Asse II salt mine) licensed to store transuranic radioactive waste for 10,00 ...
in New Mexico.
In a Presidential Memorandum dated January 29, 2010, President Obama established the Blue Ribbon Commission on America's Nuclear Future
A Blue Ribbon Commission on America's Nuclear Future was appointed by President Obama to look into future options for existing and future nuclear waste, following the ending of work on the incomplete Yucca Mountain Repository. At present, there ...
. In their final report the Commission put forth recommendations for developing a comprehensive strategy to pursue, including:
: "Recommendation #1: The United States should undertake an integrated nuclear waste management program that leads to the timely development of one or more permanent deep geological facilities for the safe disposal of spent fuel and high-level nuclear waste".
Medical experimentation
During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects. Animal studies found that a few milligrams of plutonium per kilogram of tissue is a lethal dose. In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition. This was reduced to one microgram in July 1945 after animal studies found that the way plutonium distributed itself in bones was more dangerous thanradium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
. Most of the subjects, Eileen Welsome
Eileen Welsome (born March 12, 1951) is an American journalist and author. She received a Pulitzer Prize for National Reporting in 1994 while a reporter for ''The Albuquerque Tribune'' for a 3-part story titled "The Plutonium Experiment" published ...
says, were poor, powerless, and sick.
From 1945 to 1947, eighteen human test subjects were injected with plutonium without informed consent. The tests were used to create diagnostic tools to determine the uptake of plutonium in the body in order to develop safety standards for working with plutonium. Ebb Cade was an unwilling participant in medical experiments that involved injection of 4.7 micrograms of Plutonium on 10 April 1945 at Oak Ridge, Tennessee. This experiment was under the supervision of Harold Hodge. Other experiments directed by the United States Atomic Energy Commission
The United States Atomic Energy Commission (AEC) was an agency of the United States government established after World War II by U.S. Congress to foster and control the peacetime development of atomic science and technology. President ...
and the Manhattan Project continued into the 1970s. ''The Plutonium Files
''The Plutonium Files: America's Secret Medical Experiments in the Cold War'' is a 1999 book by Eileen Welsome. It is a history of United States government-engineered radiation experiments on unwitting Americans, based on the Pulitzer Prize– ...
'' chronicles the lives of the subjects of the secret program by naming each person involved and discussing the ethical and medical research conducted in secret by the scientists and doctors. The episode is now considered to be a serious breach of medical ethics and of the Hippocratic Oath.
The government covered up most of these radiation mishaps until 1993, when President Bill Clinton
William Jefferson Clinton ( né Blythe III; born August 19, 1946) is an American politician who served as the 42nd president of the United States from 1993 to 2001. He previously served as governor of Arkansas from 1979 to 1981 and agai ...
ordered a change of policy and federal agencies then made available relevant records. The resulting investigation was undertaken by the president's Advisory Committee on Human Radiation Experiments, and it uncovered much of the material about plutonium research on humans. The committee issued a controversial 1995 report which said that "wrongs were committed" but it did not condemn those who perpetrated them.
Applications
Explosives
 The isotope plutonium-239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's
The isotope plutonium-239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's plutonium pit
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits ...
in a tamper (an optional layer of dense material) decreases the amount of plutonium needed to reach critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
by reflecting escaping neutrons back into the plutonium core. This reduces the amount of plutonium needed to reach criticality from 16 kg to 10 kg, which is a sphere with a diameter of about . This critical mass is about a third of that for uranium-235.
The Fat Man plutonium bombs used explosive compression of plutonium to obtain significantly higher densities than normal, combined with a central neutron source to begin the reaction and increase efficiency. Thus only 6.2 kg of plutonium was needed for an explosive yield equivalent to 20 kilotons of TNT.
Hypothetically, as little as 4 kg of plutonium—and maybe even less—could be used to make a single atomic bomb using very sophisticated assembly designs.
Mixed oxide fuel
Spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and ...
from normal light water reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron react ...
s contains plutonium, but it is a mixture of plutonium-242
Plutonium-242 (242Pu or Pu-242) is one of the isotopes of plutonium, the second longest-lived, with a half-life of 375,000 years.
The half-life of 242Pu is about 15 times that of 239Pu; so it is one-fifteenth as radioactive, and not one of the la ...
, 240, 239 and 238. The mixture is not sufficiently enriched for efficient nuclear weapons, but can be used once as MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an al ...
. Accidental neutron capture causes the amount of plutonium-242 and 240 to grow each time the plutonium is irradiated in a reactor with low-speed "thermal" neutrons, so that after the second cycle, the plutonium can only be consumed by fast neutron reactor
A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons (carrying energies above 1 MeV or greater, on average), as opposed ...
s. If fast neutron reactors are not available (the normal case), excess plutonium is usually discarded, and forms one of the longest-lived components of nuclear waste. The desire to consume this plutonium and other transuranic
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
fuels and reduce the radiotoxicity of the waste is the usual reason nuclear engineers give to make fast neutron reactors.
The most common chemical process, PUREX
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the ''de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium ...
(''P''lutonium–''UR''anium ''EX''traction), reprocesses spent nuclear fuel to extract plutonium and uranium which can be used to form a mixed oxide (MOX) fuel for reuse in nuclear reactors. Weapons-grade plutonium can be added to the fuel mix. MOX fuel is used in light water reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron react ...
s and consists of 60 kg of plutonium per tonne of fuel; after four years, three-quarters of the plutonium is burned (turned into other elements). Breeder reactor
A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. Breeder reactors achieve this because their neutron economy is high enough to create more fissile fuel than they use, by irradiation of a fertile mate ...
s are specifically designed to create more fissionable material than they consume.
MOX fuel has been in use since the 1980s, and is widely used in Europe. In September 2000, the United States and the Russian Federation
Russia (, , ), or the Russian Federation, is a transcontinental country spanning Eastern Europe and Northern Asia. It is the largest country in the world, with its internationally recognised territory covering , and encompassing one-eig ...
signed a Plutonium Management and Disposition Agreement The Plutonium Management and Disposition Agreement is an agreement between the United States and Russia signed in 2000, wherein both nations agreed to dispose of significant fractions of their "excess" (beyond what they need for their nuclear weapon ...
by which each agreed to dispose of 34 tonnes of weapons-grade plutonium. The U.S. Department of Energy plans to dispose of 34 tonnes of weapons-grade plutonium in the United States before the end of 2019 by converting the plutonium to a MOX fuel to be used in commercial nuclear power reactors.
MOX fuel improves total burnup. A fuel rod is reprocessed after three years of use to remove waste products, which by then account for 3% of the total weight of the rods. Any uranium or plutonium isotopes produced during those three years are left and the rod goes back into production. The presence of up to 1% gallium per mass in weapons-grade plutonium alloy has the potential to interfere with long-term operation of a light water reactor.
Plutonium recovered from spent reactor fuel poses little proliferation hazard, because of excessive contamination with non-fissile plutonium-240 and plutonium-242. Separation of the isotopes is not feasible. A dedicated reactor operating on very low burnup
In nuclear power technology, burnup (also known as fuel utilization) is a measure of how much energy is extracted from a primary nuclear fuel source. It is measured as the fraction of fuel atoms that underwent fission in %FIMA (fissions per ini ...
(hence minimal exposure of newly formed plutonium-239 to additional neutrons which causes it to be transformed to heavier isotopes of plutonium) is generally required to produce material suitable for use in efficient nuclear weapons. While "weapons-grade" plutonium is defined to contain at least 92% plutonium-239 (of the total plutonium), the United States have managed to detonate an under-20Kt device using plutonium believed to contain only about 85% plutonium-239, so called '"fuel-grade" plutonium. The "reactor-grade" plutonium produced by a regular LWR burnup cycle typically contains less than 60% Pu-239, with up to 30% parasitic Pu-240/Pu-242, and 10–15% fissile Pu-241. It is unknown if a device using plutonium obtained from reprocessed civil nuclear waste can be detonated, however such a device could hypothetically fizzle and spread radioactive materials over a large urban area. The IAEA
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 195 ...
conservatively classifies plutonium of all isotopic vectors as "direct-use" material, that is, "nuclear material that can be used for the manufacture of nuclear explosives components without transmutation or further enrichment".
Power and heat source

 The isotope plutonium-238 has a half-life of 87.74 years. It emits a large amount of
The isotope plutonium-238 has a half-life of 87.74 years. It emits a large amount of thermal energy
The term "thermal energy" is used loosely in various contexts in physics and engineering. It can refer to several different well-defined physical concepts. These include the internal energy or enthalpy of a body of matter and radiation; heat, de ...
with low levels of both gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s/photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they a ...
s and spontaneous neutron rays/particles. Being an alpha emitter, it combines high energy radiation with low penetration and thereby requires minimal shielding. A sheet of paper can be used to shield against the alpha particles emitted by plutonium-238. One kilogram of the isotope can generate about 570 watts of heat.
These characteristics make it well-suited for electrical power generation for devices that must function without direct maintenance for timescales approximating a human lifetime. It is therefore used in radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s and radioisotope heater unit
Radioisotope heater units (RHU) are small devices that provide heat through radioactive decay. They are similar to tiny radioisotope thermoelectric generators (RTG) and normally provide about one watt of heat each, derived from the decay of a fe ...
s such as those in the Cassini, Voyager, Galileo and New Horizons space probes, and the Curiosity
Curiosity (from Latin '' cūriōsitās'', from ''cūriōsus'' "careful, diligent, curious", akin to ''cura'' "care") is a quality related to inquisitive thinking such as exploration, investigation, and learning, evident by observation in humans ...
and Perseverance
Perseverance may refer to:
Behaviour
* Psychological resilience
* Perseverance of the saints, a Protestant Christian teaching
* Assurance (theology)
Geography
* Perseverance, Queensland, a locality in Australia
* Perseverance Island, Seychelles
...
(Mars 2020
Mars 2020 is a Mars rover mission forming part of NASA's Mars Exploration Program that includes the rover '' Perseverance'', the small robotic, coaxial helicopter '' Ingenuity'', and associated delivery vehicles. Mars 2020 was launched from ...
) Mars rover
A Mars rover is a motor vehicle designed to travel on the surface of Mars. Rovers have several advantages over stationary landers: they examine more territory, they can be directed to interesting features, they can place themselves in sunny pos ...
s.
The twin Voyager spacecraft were launched in 1977, each containing a 500 watt plutonium power source. Over 30 years later, each source is still producing about 300 watts which allows limited operation of each spacecraft. An earlier version of the same technology powered five Apollo Lunar Surface Experiment Packages, starting with Apollo 12 in 1969.
Plutonium-238 has also been used successfully to power artificial heart pacemakers
An artificial cardiac pacemaker (or artificial pacemaker, so as not to be confused with the natural cardiac pacemaker) or pacemaker is a medical device that generates electrical impulses delivered by electrodes to the chambers of the heart eit ...
, to reduce the risk of repeated surgery. It has been largely replaced by lithium-based primary cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction ...
s, but there were somewhere between 50 and 100 plutonium-powered pacemakers still implanted and functioning in living patients in the United States. By the end of 2007, the number of plutonium-powered pacemakers was reported to be down to just nine. Plutonium-238 was studied as a way to provide supplemental heat to scuba diving
Scuba diving is a mode of underwater diving whereby divers use breathing equipment that is completely independent of a surface air supply. The name "scuba", an acronym for " Self-Contained Underwater Breathing Apparatus", was coined by Chr ...
. Plutonium-238 mixed with beryllium is used to generate neutrons for research purposes.
Precautions
Toxicity
There are two aspects to the harmful effects of plutonium: the radioactivity and the heavy metal poison effects. Isotopes and compounds of plutonium are radioactive and accumulate in bone marrow. Contamination by plutonium oxide has resulted from nuclear disasters and radioactive incidents, including military nuclear accidents where nuclear weapons have burned. Studies of the effects of these smaller releases, as well as of the widespread radiation poisoning sickness and death following the atomic bombings of Hiroshima and Nagasaki, have provided considerable information regarding the dangers, symptoms and prognosis of radiation poisoning, which in the case of the Japanese survivors was largely unrelated to direct plutonium exposure. During the decay of plutonium, three types of ionizing radiation are released, namely alpha, beta, and gamma. Either acute or longer-term exposure carries a danger of serious health outcomes including radiation sickness,genetic damage
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mitos ...
, cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
, and death. The danger increases with the amount of exposure. Alpha radiation can travel only a short distance and cannot travel through the outer, dead layer of human skin. Beta radiation can penetrate human skin, but cannot go all the way through the body. Gamma radiation can go all the way through the body.
Even though alpha radiation cannot penetrate the skin, ingested or inhaled plutonium does irradiate internal organs. Alpha particles generated by inhaled plutonium have been found to cause lung cancer in a cohort of European nuclear workers. The skeleton, where plutonium accumulates, and the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
, where it collects and becomes concentrated, are at risk. Plutonium is not absorbed into the body efficiently when ingested; only 0.04% of plutonium oxide is absorbed after ingestion. Plutonium absorbed by the body is excreted very slowly, with a biological half-life
Biological half-life (also known as elimination half-life, pharmacologic half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the bl ...
of 200 years. Plutonium passes only slowly through cell membranes and intestinal boundaries, so absorption by ingestion and incorporation into bone structure proceeds very slowly. Donald Mastick
Donald Francis Mastick (September 1, 1920 – September 8, 2007) was an American chemist who worked at the Manhattan Project's Los Alamos Laboratory. As part of Project Alberta, he was part of the planning and preparation for the atomic bombing ...
accidentally swallowed a small amount of Plutonium(III) chloride
Plutonium(III) chloride is a chemical compound with the formula PuCl3. This ionic plutonium salt can be prepared by reacting the metal with hydrochloric acid.
Structure
Plutonium atoms in crystalline PuCl3 are 9 coordinate, and the structure is ...
, which was detectable for the next thirty years of his life, but appeared to suffer no ill effects.
Plutonium is more dangerous when inhaled than when ingested. The risk of lung cancer
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung carcinomas derive from transformed, malign ...
increases once the total radiation dose equivalent
Equivalent dose is a dose quantity '' H '' representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived from the ...
of inhaled plutonium exceeds 400 mSv. The U.S. Department of Energy estimates that the lifetime cancer risk from inhaling 5,000 plutonium particles, each about 3 µm wide, is 1% over the background U.S. average. Ingestion or inhalation of large amounts may cause acute radiation poisoning and possibly death. However, no human being is known to have died because of inhaling or ingesting plutonium, and many people have measurable amounts of plutonium in their bodies.
The "hot particle
A hot particle is a microscopic piece of radioactive material that can become lodged in living tissue and deliver a concentrated dose of radiation to a small area. A controversial theory proposes that hot particles within the body are vastly more ...
" theory in which a particle of plutonium dust irradiates a localized spot of lung tissue is not supported by mainstream research—such particles are more mobile than originally thought and toxicity is not measurably increased due to particulate form. When inhaled, plutonium can pass into the bloodstream. Once in the bloodstream, plutonium moves throughout the body and into the bones, liver, or other body organs. Plutonium that reaches body organs generally stays in the body for decades and continues to expose the surrounding tissue to radiation and thus may cause cancer.
A commonly cited quote by Ralph Nader
Ralph Nader (; born February 27, 1934) is an American political activist, author, lecturer, and attorney noted for his involvement in consumer protection, environmentalism, and government reform causes.
The son of Lebanese immigrants to the U ...
states that a pound of plutonium dust spread into the atmosphere would be enough to kill 8 billion people. This was disputed by Bernard Cohen, an opponent of the generally accepted linear no-threshold model
The linear no-threshold model (LNT) is a dose-response model used in radiation protection to estimate stochastic health effects such as radiation-induced cancer, genetic mutations and teratogenic effects on the human body due to exposure to io ...
of radiation toxicity. Cohen estimated that one pound of plutonium could kill no more than 2 million people by inhalation, so that the toxicity of plutonium is roughly equivalent with that of nerve gas
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that ...
. (Online version of Cohen's book ''The Nuclear Energy Option'' (Plenum Press, 1990) ).
Several populations of people who have been exposed to plutonium dust (e.g. people living down-wind of Nevada test sites, Nagasaki survivors, nuclear facility workers, and "terminally ill" patients injected with Pu in 1945–46 to study Pu metabolism) have been carefully followed and analyzed. Cohen found these studies inconsistent with high estimates of plutonium toxicity, citing cases such as Albert Stevens
Albert Stevens (1887–1966), also known as patient CAL-1 and most radioactive human ever, was a house painter from Ohio who was subjected to an involuntary human radiation experiment and survived the highest known accumulated radiation dose in ...
who survived into old age after being injected with plutonium. "There were about 25 workers from Los Alamos National Laboratory who inhaled a considerable amount of plutonium dust during 1940s; according to the hot-particle theory, each of them has a 99.5% chance of being dead from lung cancer by now, but there has not been a single lung cancer among them."
Marine toxicity
Investigating the toxicity of plutonium in humans is just as important as looking at the effects in fauna of marine systems. Plutonium is known to enter the marine environment by dumping of waste or accidental leakage from nuclear plants. Although the highest concentrations of plutonium in marine environments are found in the sediments, the complex biogeochemical cycle of plutonium means that it is also found in all other compartments. For example, various zooplankton species that aid in thenutrient cycle
A nutrient cycle (or ecological recycling) is the movement and exchange of inorganic and organic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyc ...
will consume the element on a daily basis. The complete excretion of ingested plutonium by zooplankton makes their defecation an extremely important mechanism in the scavenging of plutonium from surface waters. However, those zooplankton that succumb to predation by larger organisms may become a transmission vehicle of plutonium to fish.
In addition to consumption, fish can also be exposed to plutonium by their geographical distribution around the globe. One study investigated the effects of transuranium elements (plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suita ...
, plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
, plutonium-240
Plutonium-240 ( or Pu-240) is an isotope of plutonium formed when plutonium-239 captures a neutron. The detection of its spontaneous fission led to its discovery in 1944 at Los Alamos and had important consequences for the Manhattan Project.
240 ...
) on various fish living in the Chernobyl Exclusion Zone
The Chernobyl Nuclear Power Plant Zone of Alienation, Belarusian: Хона адчужэння Чарнобыльскай АЭС, ''Zona adčužennia Čarnobyĺskaj AES'', russian: Зона отчуждения Чернобыльской АЭС, ...
(CEZ). Results showed that a proportion of female perch in the CEZ displayed either a failure or delay in maturation of the gonads. Similar studies found large accumulations of plutonium in the respiratory and digestive organs of cod, flounder and herring.
Plutonium toxicity is just as detrimental to larvae of fish in nuclear waste areas. Undeveloped eggs have a higher risk than developed adult fish exposed to the element in these waste areas. The Oak Ridge National Laboratory displayed that carp and minnow embryos raised in solutions containing plutonium isotopes did not hatch; eggs that hatched displayed significant abnormalities when compared to control developed embryos. It revealed that higher concentrations of plutonium have been found to cause issues in marine fauna exposed to the element.
Criticality potential
 Care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235. A critical mass of plutonium emits lethal amounts of neutrons and
Care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium-235. A critical mass of plutonium emits lethal amounts of neutrons and gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s. Plutonium in solution is more likely to form a critical mass than the solid form due to moderation
Moderation is the process of eliminating or lessening extremes. It is used to ensure normality throughout the medium on which it is being conducted. Common uses of moderation include:
*Ensuring consistency and accuracy in the marking of stud ...
by the hydrogen in water.
Criticality accident
A criticality accident is an accidental uncontrolled nuclear fission chain reaction. It is sometimes referred to as a critical excursion, critical power excursion, or divergent chain reaction. Any such event involves the unintended accumulation ...
s have occurred in the past, some of them with lethal consequences. Careless handling of tungsten carbide
Tungsten carbide (chemical formula: WC) is a chemical compound (specifically, a carbide) containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into ...
bricks around a 6.2 kg plutonium sphere resulted in a fatal dose of radiation at Los Alamos on August 21, 1945, when scientist Harry Daghlian
Haroutune Krikor Daghlian Jr. (May 4, 1921 – September 15, 1945) was an American physicist with the Manhattan Project, which designed and produced the atomic bombs that were used in World War II. He accidentally irradiated himself on August ...
received a dose estimated to be 5.1 sievert (510 rems) and died 25 days later. Nine months later, another Los Alamos scientist, Louis Slotin
Louis Alexander Slotin (1 December 1910 – 30 May 1946) was a Canadian physicist and chemist who took part in the Manhattan Project. Born and raised in the North End of Winnipeg, Manitoba, Slotin earned both his Bachelor of Science and M ...
, died from a similar accident involving a beryllium reflector and the same plutonium core (the so-called "demon core
The demon core was a spherical subcritical mass of plutonium in diameter, manufactured during World War II by the United States nuclear weapon development effort, the Manhattan Project, as a fissile core for an early atomic bomb. The core w ...
") that had previously claimed the life of Daghlian.
In December 1958, during a process of purifying plutonium at Los Alamos, a critical mass was formed in a mixing vessel, which resulted in the death of a chemical operator named Cecil Kelley. Other nuclear accidents
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, lar ...
have occurred in the Soviet Union, Japan, the United States, and many other countries.
Flammability
Metallic plutonium is a fire hazard, especially if the material is finely divided. In a moist environment, plutonium forms hydrides on its surface, which are pyrophoric and may ignite in air at room temperature. Plutonium expands up to 70% in volume as it oxidizes and thus may break its container. The radioactivity of the burning material is an additional hazard.Magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions ...
sand is probably the most effective material for extinguishing a plutonium fire. It cools the burning material, acting as a heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is dissipated away from the device, th ...
, and also blocks off oxygen. Special precautions are necessary to store or handle plutonium in any form; generally a dry inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
atmosphere is required.
Transportation
Land and sea
The usual transportation of plutonium is through the more stable plutonium oxide in a sealed package. A typical transport consists of one truck carrying one protected shipping container, holding a number of packages with a total weight varying from 80 to 200 kg of plutonium oxide. A sea shipment may consist of several containers, each of them holding a sealed package. The United States Nuclear Regulatory Commission dictates that it must be solid instead of powder if the contents surpass 0.74 TBq (20 Curies) of radioactive activity. In 2016, the ships ''Pacific Egret'' and ''Pacific Heron'' of Pacific Nuclear Transport Ltd. transported 331 kg (730 lbs) of plutonium to a United States government facility in Savannah River,South Carolina
)'' Animis opibusque parati'' ( for, , Latin, Prepared in mind and resources, links=no)
, anthem = " Carolina";" South Carolina On My Mind"
, Former = Province of South Carolina
, seat = Columbia
, LargestCity = Charleston
, LargestMetro = ...
.
Air
The U.S. Government air transport regulations permit the transport of plutonium by air, subject to restrictions on other dangerous materials carried on the same flight, packaging requirements, and stowage in the rearmost part of the aircraft. In 2012 media revealed that plutonium has been flown out of Norway on commercialpassenger airline
An airline is a company that provides air transport services for traveling passengers and freight. Airlines use aircraft to supply these services and may form partnerships or alliances with other airlines for codeshare agreements, in whic ...
s—around every other year—including one time in 2011. Regulations permit an airplane to transport 15 grams of fissionable material. Such plutonium transportation is without problems, according to a senior advisor (''seniorrådgiver'') at Statens strålevern.
Notes
Footnotes
Citations
References
* * * * * * * * * * * * * * * * * * * * * * * * * * *External links
* * * * * * * * * * * * {{featured article Chemical elements Actinides Carcinogens Nuclear materials Synthetic elements Manhattan Project Materials that expand upon freezing