|
Primary Cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and not recharged with electricity and reused like a secondary cell (rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants. Primary cells are made in a range of standard sizes to power small household appliances such as flashlights and portable radios. Primary batteries make up about 90% of the $50 billion battery market, but secondary batteries have been gaining market share. About 15 billion primary batteries are thrown away worldwide every year, vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Batteries Comparison 4,5 D C AA AAA AAAA A23 9V CR2032 LR44 Matchstick-1
Battery most often refers to: * Electric battery, a device that provides electrical power * Battery (crime), a crime involving unlawful physical contact Battery may also refer to: Energy source *Automotive battery, a device to provide power to certain functions of an automobile *List of battery types *Energy storage, including batteries that are not electrochemical Law * Battery (tort), a civil wrong in common law of intentional harmful or offensive contact Military and naval uses * Artillery battery, an organized group of artillery pieces ** Main battery, the primary weapons of a warship ** Secondary battery (artillery), the smaller guns on a warship * Battery, a position of a cartridge in a firearm action Arts and entertainment Music * Battery (electro-industrial band) * Battery (hardcore punk band) * "Battery", a song by Metallica from the 1986 album ''Master of Puppets'' * Marching percussion ensemble, frequently known as a battery * Battery, a software music sampler b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rechargeable Batteries
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer (Li-ion polymer). Rechargeable batteries typically initially cost more than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
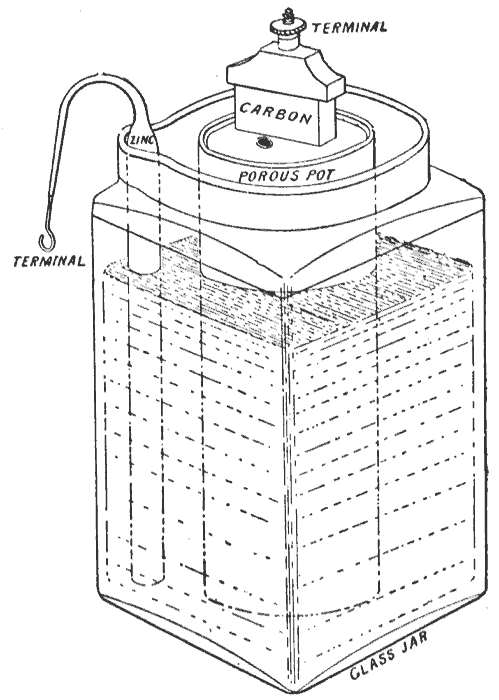
Grove Cell
The Grove cell was an early electric primary cell named after its inventor, Welsh physical scientist William Robert Grove, and consisted of a zinc anode in dilute sulfuric acid and a platinum cathode in concentrated nitric acid, the two separated by a porous ceramic pot. Cell details The Grove cell voltage is about 1.9 volts and arises from the following reaction: : Zn + H2SO4 + 2 HNO3 ZnSO4 + 2 H2O + 2 NO2↑ Use The Grove cell was the favored power source of the early American telegraph system in the period 1840 – 1860 because it offered a high current output and higher voltage than the earlier Daniell cell (at 1.9 volts and 1.1 volts, respectively). Disadvantages By the time of the American Civil War, as telegraph traffic increased, the Grove cell's tendency to discharge poisonous nitrogen dioxide (NO2) fumes proved increasingly hazardous to health, and as telegraphs became more complex, the need for constant voltage became critical. The Grove cell was limited in this respe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bunsen Cell
The Bunsen cell is a zinc-carbon primary cell (colloquially called a "battery") composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in Nitric acid, nitric or chromic acid. Cell details The Bunsen cell is about 1.9 volts and arises from the following reaction: : Zn + H2SO4 + 2 HNO3 ZnSO4 + 2 H2O + 2 NO2(g) According to the reaction above, when 1 mole (unit), mole (or part) each of zinc and sulfuric acid react with 2 moles (or parts) of nitric acid, the resultant products formed are, 1 mole (or part) of zinc sulfate and 2 moles (or parts) each of water and nitrogen dioxide (gaseous, in the form of bubbles). The cell is named after its inventor, German chemist Robert Wilhelm Bunsen, who improved upon the Grove cell by replacing William Robert Grove, Grove's expensive platinum cathode with carbon in the form of pulverized coal and coke (fuel), coke. Like Grove's battery, Bunsen's emitted noxious fumes of nitrogen dioxide. Bunsen use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leclanché Cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell. History In 1866, Georges Leclanché invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts.Simms, J.W. (1965) ''The Boy Electrician'' M.I.E.E. p. 61 This cell achieved very quick success in telegraphy, signalling and electric bell work. The dry cell form was used to power early telephones—usually from an adjacent woo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. is α polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (this polymorph is called pyrolusite or ), with three-coordinate oxide and octahedral metal centres. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an Redox, oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Munitions
Ammunition (informally ammo) is the material fired, scattered, dropped, or detonated from any weapon or weapon system. Ammunition is both expendable weapons (e.g., bombs, missiles, grenades, land mines) and the component parts of other weapons that create the effect on a target (e.g., bullets and warheads). The purpose of ammunition is to project a force against a selected target to have an effect (usually, but not always, lethal). An example of ammunition is the firearm cartridge, which includes all components required to deliver the weapon effect in a single package. Until the 20th century, black powder was the most common propellant used but has now been replaced in nearly all cases by modern compounds. Ammunition comes in a great range of sizes and types and is often designed to work only in specific weapons systems. However, there are internationally recognized standards for certain ammunition types (e.g., 5.56×45mm NATO) that enable their use across different weapo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |