|
Annealing (metallurgy)
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling. In annealing, atoms migrate in the crystal lattice and the number of dislocations decreases, leading to a change in ductility and hardness. As the material cools it recrystallizes. For many alloys, including carbon steel, the crystal grain size and phase composition, which ultimately determine the material properties, are dependent on the heating rate and cooling rate. Hot working or cold working after the annealing process alters the metal structure, so further heat treatments may be used to achieve the properties required. With knowledge of the composition and phase diagram, heat treatment can be used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals; that is, the way in which science is applied to the production of metals, and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist. The science of metallurgy is further subdivided into two broad categories: chemical metallurgy and physical metallurgy. Chemical metallurgy is chiefly concerned with the reduction and oxidation of metals, and the chemical performance of metals. Subjects of study in chemical metallurgy inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arrhenius Equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 1884 that the van 't Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. This equation has a vast and important application in determining the rate of chemical reactions and for calculation of energy of activation. Arrhenius provided a physical justification and interpretation for the formula. Laidler, K. J. (1987) ''Chemical Kinetics'', Third Edition, Harper & Row, p. 42 Currently, it is best seen as an empirical relationship.Kenneth Connors, Chemical Kinetics, 1990, VCH Publishers It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnet
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets. A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Forming Gas
Forming gas is a mixture of hydrogen (mole fraction varies) and nitrogen. It is sometimes called a "dissociated ammonia atmosphere" due to the reaction which generates it: :2 NH3 → 3 H2 + N2 It can also be manufactured by thermal cracking of ammonia, in an ammonia cracker or forming gas generator. Forming gas is used as an atmosphere for processes that need the properties of hydrogen gas. Typical forming gas formulations (5% H2 in N2) are not explosive. It is used in chambers for gas hypersensitization, a process in which photographic film is heated in forming gas to drive out moisture and oxygen and to increase the base fog of the film. Hypersensitization is used particularly in deep-sky astrophotography, which deals with low-intensity incoming light, requires long exposure times, and is thus particularly sensitive to contaminants in the film. Forming gas is also used to regenerate catalysts in glove boxes and as an atmosphere for annealing processes. It can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Gas
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Gas
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
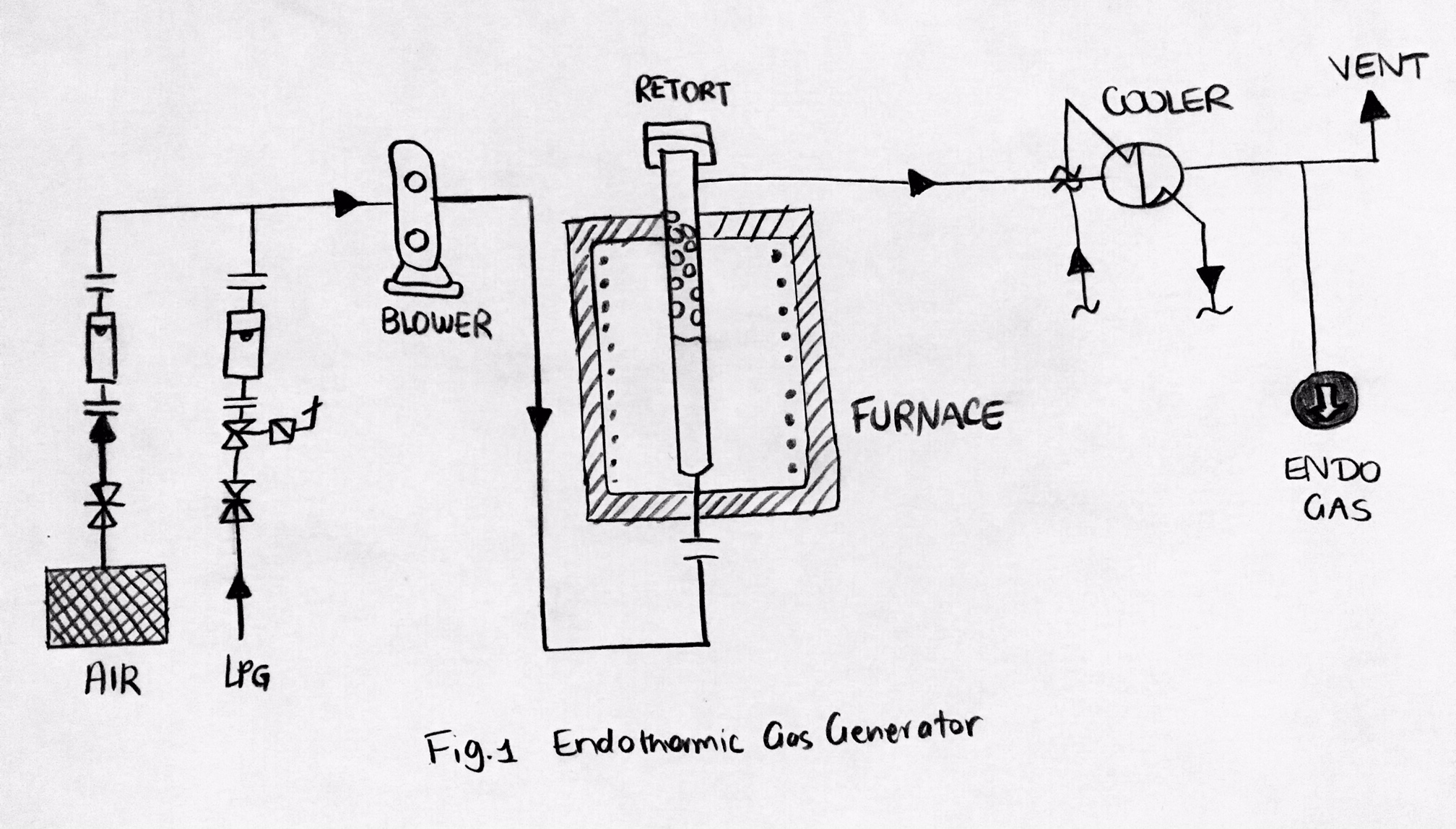
Endothermic Gas
Endothermic gas is a gas that inhibits or reverses oxidation on the surfaces it is in contact with. This gas is the product of incomplete combustion in a controlled environment. An example mixture is hydrogen gas (H2), nitrogen gas (N2), and carbon monoxide (CO). The hydrogen and carbon monoxide are reducing agents, so they work together to shield surfaces from oxidation. Endothermic gas is often used as a carrier gas for gas carburizing and carbonitriding. An endothermic gas generator could be used to supply heat to form an endothermic reaction. Synthesised in the catalytic retort(s) of endothermic generators, the gas in the endothermic atmosphere is combined with an additive gas including natural gas, propane (C3H8) or air and is then used to improve the surface chemistry work positioned in the furnace. Purposes There are two common purposes of the atmospheres in the heat treating industry: # Protect the processed material from surface reactions (chemically inert) # Allow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosphere is the outer region of a star, which includes the layers above the opaque photosphere; stars of low temperature might have outer atmospheres containing compound molecules. The atmosphere of Earth is composed of nitrogen (78%), oxygen (21%), argon (0.9%), carbon dioxide (0.04%) and trace gases. Most organisms use oxygen for respiration; lightning and bacteria perform nitrogen fixation to produce ammonia that is used to make nucleotides and amino acids; plants, algae, and cyanobacteria use carbon dioxide for photosynthesis. The layered composition of the atmosphere minimises the harmful effects of sunlight, ultraviolet radiation, the solar wind, and cosmic rays to protect organisms from genetic damage. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hardening (metallurgy)
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. A harder metal will have a higher resistance to plastic deformation than a less hard metal. Processes The five hardening processes are: *The Hall–Petch method, or grain boundary strengthening, is to obtain small grains. Smaller grains increases the likelihood of dislocations running into grain boundaries after shorter distances, which are very strong dislocation barriers. In general, smaller grain size will make the material harder. When the grain size approach sub-micron sizes, some materials may however become softer. This is simply an effect of another deformation mechanism that becomes easier, i.e. grain boundary sliding. At this point, all dislocation related hardening mechanisms become irrelevant. *In work hardening (also referred to as strain hardening) the ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grain Growth
In materials science, grain growth is the increase in size of grains (crystallites) in a material at high temperature. This occurs when recovery and recrystallisation are complete and further reduction in the internal energy can only be achieved by reducing the total area of grain boundary. The term is commonly used in metallurgy but is also used in reference to ceramics and minerals. The behaviors of grain growth is analogous to the coarsening behaviors of grains, which implied that both of grain growth and coarsening may be dominated by the same physical mechanism. Importance of grain growth The practical performances of polycrystalline materials are strongly affected by the formed microstructure inside, which is mostly dominated by grain growth behaviors. For example, most materials exhibit the Hall–Petch effect at room-temperature and so display a higher yield stress when the grain size is reduced (assuming abnormal grain growth has not taken place). At high temperatures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) below 0°C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phases at continuous phase transitions start to form im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |