Particulate Inorganic Carbon on:
[Wikipedia]
[Google]
[Amazon]
 Particulate inorganic carbon (PIC) can be contrasted with
Particulate inorganic carbon (PIC) can be contrasted with

Chapter 12: Ocean Sediments
page 273–297, Rebus Community. Updated 2020.
 The
The
"The calcium carbonate counter pump: Fundamentals, evolution through time, and future feedbacks"
''American Geophysical Union'', pp.B23A-08.
 An
An
 A
A

 Since the industrial revolution 30% of the anthropogenic CO2 has been absorbed by the oceans, resulting in
Since the industrial revolution 30% of the anthropogenic CO2 has been absorbed by the oceans, resulting in  Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License

Protist shell
Many protists have protective shells or tests, usually made from silica (glass) or calcium carbonate (chalk). Protists are mostly single-celled and microscopic. Their shells are often tough, mineralised forms that resist degradation, and can ...
s">
File:Coccolithus pelagicus.jpg, ''
 Particulate inorganic carbon (PIC) can be contrasted with
Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic c ...
(DIC), the other form of inorganic carbon
Total inorganic carbon (''C''T or TIC) is the sum of the inorganic carbon species.
Carbon Chemical compound, compounds can be distinguished as either Organic compound, organic or inorganic, and Dissolved organic carbon, dissolved or Particulate ...
found in the ocean. These distinctions are important in chemical oceanography
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field ...
. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.
Most PIC is calcium carbonate, CaCO3, particularly in the form of calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, but also in the form of aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including prec ...
. Calcium carbonate makes up the shells of many marine organism
Marine life, sea life, or ocean life is the aquatic plant, plants, aquatic animal, animals and other organisms that live in the seawater, salt water of seas or oceans, or the brackish water of coastal estuary, estuaries. At a fundamental leve ...
s. It also forms during whiting event
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. Th ...
s and is excreted by marine fish during osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
.
Overview
Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as –protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s, lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include ...
s, carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ma ...
s, and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, HCO3−, CO32− respectively).
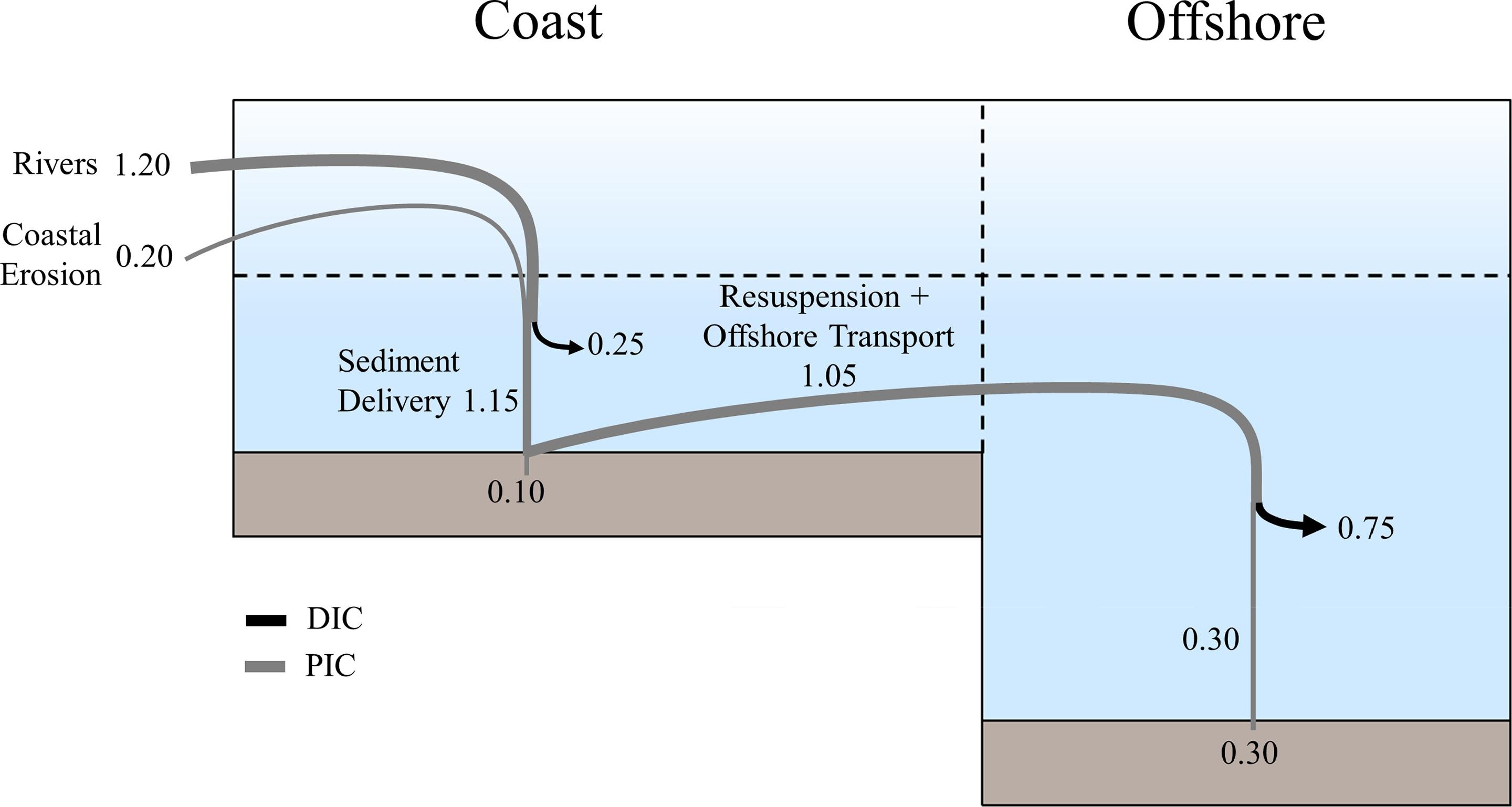
Marine carbon is further separated into particulate and dissolved phases. These pools are operationally defined by physical separation – dissolved carbon passes through a 0.2 μm filter, and particulate carbon does not.
There are two main types of inorganic carbon that are found in the oceans. Dissolved inorganic carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic c ...
(DIC) is made up of bicarbonate (HCO3−), carbonate (CO32−) and carbon dioxide (including both dissolved CO2 and carbonic acid H2CO3). DIC can be converted to particulate inorganic carbon (PIC) through precipitation of CaCO3 (biologically or abiotically). DIC can also be converted to particulate organic carbon (POC) through photosynthesis and chemoautotrophy
A Chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic ( chemoorganotrophs) or inorganic (chemolithotrophs). The chemotroph designation is in contrast to phototrop ...
(i.e. primary production). DIC increases with depth as organic carbon particles sink and are respired. Free oxygen decreases as DIC increases because oxygen is consumed during aerobic respiration.
Particulate inorganic carbon (PIC) is the other form of inorganic carbon found in the ocean. Most PIC is the CaCO3 that makes up shells of various marine organisms, but can also form in whiting event
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. Th ...
s. Marine fish also excrete calcium carbonate during osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
.
Some of the inorganic carbon species in the ocean, such as bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
and carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
, are major contributors to alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of ...
, a natural ocean buffer that prevents drastic changes in acidity (or pH). The marine carbon cycle also affects the reaction and dissolution rates of some chemical compounds, regulates the amount of carbon dioxide in the atmosphere and Earth's temperature.


Calcium carbonate
Particulate inorganic carbon (PIC) usually takes the form of calcium carbonate (CaCO3), and plays a key part in the ocean carbon cycle. This biologically fixed carbon is used as a protective coating for many planktonic species (coccolithophores, foraminifera) as well as larger marine organisms (mollusk shells). Calcium carbonate is also excreted at high rates duringosmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
by fish, and can form in whiting event
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. Th ...
s. While this form of carbon is not directly taken from the atmospheric budget, it is formed from dissolved forms of carbonate which are in equilibrium with CO2 and then responsible for removing this carbon via sequestration.
:CO2 + H2O → H2CO3 → H+ + HCO3−
:Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O
While this process does manage to fix a large amount of carbon, two units of alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of ...
are sequestered for every unit of sequestered carbon. The formation and sinking of CaCO3 therefore drives a surface to deep alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of ...
gradient which serves to raise the pH of surface waters, shifting the speciation of dissolved carbon to raise the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
of dissolved CO2 in surface waters, which actually raises atmospheric levels. In addition, the burial of CaCO3 in sediments serves to lower overall oceanic alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of ...
, tending to raise pH and thereby atmospheric CO2 levels if not counterbalanced by the new input of alkalinity from weathering.Sigman DM & GH Haug. 2006. The biological pump in the past. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 491-528 The portion of carbon that is permanently buried at the sea floor becomes part of the geologic record. Calcium carbonate often forms remarkable deposits that can then be raised onto land through tectonic motion as in the case with the White Cliffs of Dover
The White Cliffs of Dover is the region of English coastline facing the Strait of Dover and France. The cliff face, which reaches a height of , owes its striking appearance to its composition of chalk accented by streaks of black flint, deposi ...
in Southern England. These cliffs are made almost entirely of the plates of buried coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s.Webb, Paul (2019) ''Introduction to Oceanography''Chapter 12: Ocean Sediments
page 273–297, Rebus Community. Updated 2020.
Carbonate pump
 The
The carbonate pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments.Sigman DM & GH ...
, sometimes called the carbonate counter pump, starts with marine organisms at the ocean's surface producing particulate inorganic carbon (PIC) in the form of calcium carbonate (calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
or aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including prec ...
, CaCO3). This CaCO3 is what forms hard body parts like shells. The formation of these shells increases atmospheric CO2 due to the production of CaCO3 in the following reaction with simplified stoichiometry:Coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s, a nearly ubiquitous group of phytoplankton that produce shells of calcium carbonate, are the dominant contributors to the carbonate pump. Due to their abundance, coccolithophores have significant implications on carbonate chemistry, in the surface waters they inhabit and in the ocean below: they provide a large mechanism for the downward transport of CaCO3. The air-sea CO2 flux induced by a marine biological community
A community is a social unit (a group of living things) with commonality such as place, norms, religion, values, customs, or identity. Communities may share a sense of place situated in a given geographical area (e.g. a country, village, tow ...
can be determined by the rain ratio - the proportion of carbon from calcium carbonate compared to that from organic carbon in particulate matter sinking to the ocean floor, (PIC/POC). The carbonate pump acts as a negative feedback on CO2 taken into the ocean by the solubility pump. It occurs with lesser magnitude than the solubility pump.
The carbonate pump is sometimes referred to as the "hard tissue" component of the biological pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments.Sigman DM & GH ...
. Some surface marine organisms, like coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s, produce hard structures out of calcium carbonate, a form of particulate inorganic carbon, by fixing bicarbonate. This fixation of DIC is an important part of the oceanic carbon cycle.
:Ca2+ + 2 HCO3− → CaCO3 + CO2 + H2O
While the biological carbon pump fixes inorganic carbon (CO2) into particulate organic carbon
Particulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.053 to 2 millimeters.
Particulate organic carbon (POC) is ...
in the form of sugar (C6H12O6), the carbonate pump fixes inorganic bicarbonate and causes a net release of CO2. In this way, the carbonate pump could be termed the carbonate counter pump. It works counter to the biological pump by counteracting the CO2 flux from the biological pump.Zeebe, R.E., 2016"The calcium carbonate counter pump: Fundamentals, evolution through time, and future feedbacks"
''American Geophysical Union'', pp.B23A-08.
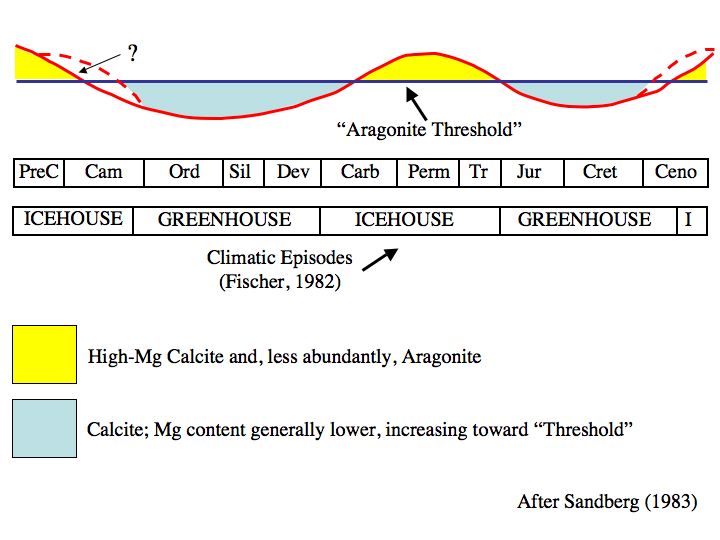
Calcite and aragonite seas
 An
An aragonite sea
An aragonite sea contains aragonite and high-magnesium calcite as the primary inorganic calcium carbonate precipitates. The chemical conditions of the seawater must be notably high in magnesium content relative to calcium (high Mg/Ca ratio) for ...
contains aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including prec ...
and high-magnesium calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
as the primary inorganic calcium carbonate precipitates. The chemical conditions of the seawater must be notably high in magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
content relative to calcium (high Mg/Ca ratio) for an aragonite sea to form. This is in contrast to a calcite sea
A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic ca ...
in which seawater low in magnesium content relative to calcium (low Mg/Ca ratio) favors the formation of low-magnesium calcite as the primary inorganic marine calcium carbonate precipitate.
The Early Paleozoic
The Paleozoic (or Palaeozoic) Era is the earliest of three geologic eras of the Phanerozoic Eon.
The name ''Paleozoic'' ( ;) was coined by the British geologist Adam Sedgwick in 1838
by combining the Greek words ''palaiós'' (, "old") and ' ...
and the Middle to Late Mesozoic
The Mesozoic Era ( ), also called the Age of Reptiles, the Age of Conifers, and colloquially as the Age of the Dinosaurs is the second-to-last era of Earth's geological history, lasting from about , comprising the Triassic, Jurassic and Cretaceo ...
oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic
The Cenozoic ( ; ) is Earth's current geological era, representing the last 66million years of Earth's history. It is characterised by the dominance of mammals, birds and flowering plants, a cooling and drying climate, and the current configura ...
(including today) are characterized by aragonite seas.
Aragonite seas occur due to several factors, the most obvious of these is a high seawater Mg/Ca ratio (Mg/Ca > 2), which occurs during intervals of slow seafloor spreading
Seafloor spreading or Seafloor spread is a process that occurs at mid-ocean ridges, where new oceanic crust is formed through volcanic activity and then gradually moves away from the ridge.
History of study
Earlier theories by Alfred Wegener an ...
. However, the sea level
Mean sea level (MSL, often shortened to sea level) is an average surface level of one or more among Earth's coastal bodies of water from which heights such as elevation may be measured. The global MSL is a type of vertical datuma standardised g ...
, temperature, and calcium carbonate saturation state of the surrounding system also determine which polymorph of calcium carbonate (aragonite, low-magnesium calcite, high-magnesium calcite) will form.
Likewise, the occurrence of calcite seas is controlled by the same suite of factors controlling aragonite seas, with the most obvious being a low seawater Mg/Ca ratio (Mg/Ca < 2), which occurs during intervals of rapid seafloor spreading.
Whiting events
 A
A whiting event
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. Th ...
is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies
A body of water or waterbody (often spelled water body) is any significant accumulation of water on the surface of Earth or another planet. The term most often refers to oceans, seas, and lakes, but it includes smaller pools of water such as p ...
, typically during summer months, as a result of photosynthetic
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in c ...
microbiological activity or sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sand an ...
disturbance. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters. They can also occur in both marine and freshwater environments. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words (), meaning 'plant', and (), meaning 'wanderer' or 'drifter'.
Ph ...
. Because whiting events affect aquatic chemistry, physical properties, and carbon cycling
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component ...
, studying the mechanisms behind them holds scientific relevance in various ways.
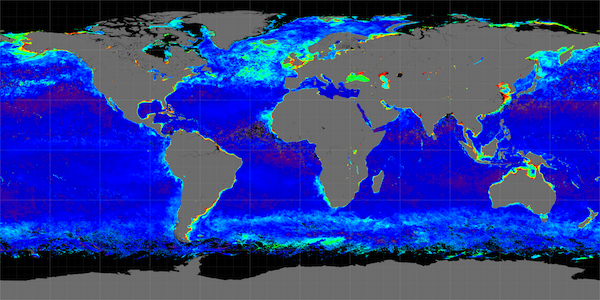
Great Calcite Belt
The Great Calcite Belt (GCB) of theSouthern Ocean
The Southern Ocean, also known as the Antarctic Ocean, comprises the southernmost waters of the World Ocean, generally taken to be south of 60° S latitude and encircling Antarctica. With a size of , it is regarded as the second-small ...
is a region of elevated summertime upper ocean calcite concentration derived from coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s, despite the region being known for its diatom
A diatom (Neo-Latin ''diatoma''), "a cutting through, a severance", from el, διάτομος, diátomos, "cut in half, divided equally" from el, διατέμνω, diatémno, "to cut in twain". is any member of a large group comprising sev ...
predominance. The overlap of two major phytoplankton groups, coccolithophores and diatoms, in the dynamic frontal systems characteristic of this region provides an ideal setting to study environmental
influences on the distribution of different species within these taxonomic groups.
The Great Calcite Belt, defined as an elevated particulate inorganic carbon (PIC) feature occurring alongside seasonally elevated chlorophyll a in austral spring and summer in the Southern Ocean, plays an important role in climate fluctuations, accounting for over 60% of the Southern Ocean area (30–60° S). The region between 30° and 50° S has the highest uptake of anthropogenic carbon dioxide (CO2) alongside the North Atlantic and North Pacific oceans. Knowledge of the impact of interacting environmental influences on phytoplankton distribution in the Southern Ocean is limited. For example, more understanding is needed of how light and iron availability or temperature and pH interact to control phytoplankton biogeography
Biogeography is the study of the distribution of species and ecosystems in geographic space and through geological time. Organisms and biological communities often vary in a regular fashion along geographic gradients of latitude, elevation, ...
. Hence, if model parameterizations are to improve to provide accurate predictions of biogeochemical change, a multivariate understanding of the full suite of environmental drivers is required.
The Southern Ocean has often been considered as a microplankton-dominated (20–200 µm) system with phytoplankton bloom
An algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in freshwater or marine water systems. It is often recognized by the discoloration in the water from the algae's pigments. The term ''algae'' encompass ...
s dominated by large diatoms and ''Phaeocystis
''Phaeocystis'' is a genus of algae belonging to the Prymnesiophyte class and to the larger division of Haptophyta. It is a widespread marine phytoplankton and can function at a wide range of temperatures (eurythermal) and salinities (euryhalin ...
'' sp. However, since the identification of the GCB as a consistent feature and the recognition of picoplankton
Picoplankton is the fraction of plankton composed by cells between 0.2 and 2 μm that can be either prokaryotic and eukaryotic phototrophs and heterotrophs:
* photosynthetic
* heterotrophic
They are prevalent amongst microbial plankton communit ...
(< 2 µm) and nanoplankton
Plankton are the diverse collection of organisms found in water (or air) that are unable to propel themselves against a current (or wind). The individual organisms constituting plankton are called plankters. In the ocean, they provide a crucia ...
(2–20 µm) importance in high-nutrient, low-chlorophyll
High-nutrient, low-chlorophyll (HNLC) regions are regions of the ocean where the abundance of phytoplankton is low and fairly constant despite the availability of macronutrients. Phytoplankton rely on a suite of nutrients for cellular function. Ma ...
(HNLC) waters, the dynamics of small (bio)mineralizing plankton and their export need to be acknowledged. The two dominant biomineralizing phytoplankton groups in the GCB are coccolithophores and diatoms. Coccolithophores are generally found north of the polar front, though ''Emiliania huxleyi
''Emiliania huxleyi'' is a species of coccolithophore found in almost all ocean ecosystems from the equator to sub-polar regions, and from nutrient rich upwelling zones to nutrient poor oligotrophic waters. It is one of thousands of different ...
'' has been observed as far south as 58° S in the Scotia Sea
The Scotia Sea is a sea located at the northern edge of the Southern Ocean at its boundary with the South Atlantic Ocean. It is bounded on the west by the Drake Passage and on the north, east, and south by the Scotia Arc, an undersea ridge and i ...
, at 61° S across Drake Passage
The Drake Passage (referred to as Mar de Hoces Hoces Sea"in Spanish-speaking countries) is the body of water between South America's Cape Horn, Chile and the South Shetland Islands of Antarctica. It connects the southwestern part of the Atla ...
, and at 65°S south of Australia.
Diatoms are present throughout the GCB, with the polar front
In meteorology, the polar front is the weather front boundary between the polar cell and the Ferrel cell around the 60° latitude, near the polar regions, in both hemisphere. At this boundary a sharp gradient in temperature occurs between these ...
marking a strong divide between different size fractions. North of the polar front, small diatom species, such as ''Pseudo-nitzschia
''Pseudo-nitzschia'' is a marine planktonic diatom genus that accounts for 4.4% of pennate diatoms found worldwide. Some species are capable of producing the neurotoxin domoic acid (DA), which is responsible for the neurological disorder in human ...
'' spp. and ''Thalassiosira
''Thalassiosira'' is a genus of centric diatoms, comprising over 100 marine and freshwater species. It is a diverse group of photosynthetic eukaryotes that make up a vital part of marine and freshwater ecosystems, in which they are key primary pr ...
'' spp., tend to dominate numerically, whereas large diatoms with higher silicic acid
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
requirements (e.g., ''Fragilariopsis kerguelensis
''Fragilariopsis kerguelensis'', is a pennate diatom native to the Southern Ocean. It has been characterized as "the most abundant diatom in the Antarctic Seas".
Description
''Fragilariopsis kerguelensis'' is a unicellular, phototrophic, micro ...
'') are generally more abundant south of the polar front. High abundances of nanoplankton (coccolithophores, small diatoms, chrysophyte
The Chrysophyceae, usually called chrysophytes, chrysomonads, golden-brown algae or golden algae are a large group of algae, found mostly in freshwater. Golden algae is also commonly used to refer to a single species, ''Prymnesium parvum'', which ...
s) have also been observed on the Patagonian Shelf
The Argentine (sometimes referred to as Patagonian) Shelf is part of the South American continental shelf belonging to the Argentine Sea on the Atlantic seaboard, south of about 35°S. It adjoins the coasts of Uruguay, Argentina and the Falkla ...
and in the Scotia Sea
The Scotia Sea is a sea located at the northern edge of the Southern Ocean at its boundary with the South Atlantic Ocean. It is bounded on the west by the Drake Passage and on the north, east, and south by the Scotia Arc, an undersea ridge and i ...
. Currently, few studies incorporate small biomineralizing phytoplankton to species level. Rather, the focus has often been on the larger and noncalcifying species in the Southern Ocean due to sample preservation issues (i.e., acidified Lugol’s solution dissolves calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
, and light microscopy restricts accurate identification to cells > 10 µm. In the context of climate change and future ecosystem function, the distribution of biomineralizing phytoplankton is important to define when considering phytoplankton interactions with carbonate chemistry, and ocean biogeochemistry.
The Great Calcite Belt spans the major Southern Ocean circumpolar fronts: the Subantarctic front, the polar front, the Southern Antarctic Circumpolar Current front, and occasionally the southern boundary of the Antarctic Circumpolar Current
The Antarctic Circumpolar Current (ACC) is an ocean current that flows clockwise (as seen from the South Pole) from west to east around Antarctica. An alternative name for the ACC is the West Wind Drift. The ACC is the dominant circulation feat ...
. The subtropical front
A subtropical front is a surface water mass boundary or front, which is a narrow zone of transition between air masses of contrasting density, air masses of different temperatures or different water vapour concentrates.
It is also characterized by ...
(at approximately 10 °C) acts as the northern boundary of the GCB and is associated with a sharp increase in PIC southwards. These fronts divide distinct environmental and biogeochemical zones, making the GCB an ideal study area to examine controls on phytoplankton communities in the open ocean. A high PIC concentration observed in the GCB (1 µmol PIC L−1) compared to the global average (0.2 µmol PIC L−1) and significant quantities of detached ''E. huxleyi'' coccoliths (in concentrations > 20,000 coccoliths mL−1) both characterize the GCB. The GCB is clearly observed in satellite imagery
Satellite images (also Earth observation imagery, spaceborne photography, or simply satellite photo) are images of Earth collected by imaging satellites operated by governments and businesses around the world. Satellite imaging companies sell ima ...
spanning from the Patagonian Shelf
The Argentine (sometimes referred to as Patagonian) Shelf is part of the South American continental shelf belonging to the Argentine Sea on the Atlantic seaboard, south of about 35°S. It adjoins the coasts of Uruguay, Argentina and the Falkla ...
across the Atlantic, Indian, and Pacific oceans and completing Antarctic circumnavigation via the Drake Passage.

Coccolithophores
 Since the industrial revolution 30% of the anthropogenic CO2 has been absorbed by the oceans, resulting in
Since the industrial revolution 30% of the anthropogenic CO2 has been absorbed by the oceans, resulting in ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
, which is a threat to calcifying alga
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mi ...
. As a result, there has been profound interest in these calcifying algae, boosted by their major role in the global carbon cycle. Globally, coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdo ...
s, particularly ''Emiliania huxleyi
''Emiliania huxleyi'' is a species of coccolithophore found in almost all ocean ecosystems from the equator to sub-polar regions, and from nutrient rich upwelling zones to nutrient poor oligotrophic waters. It is one of thousands of different ...
'', are considered to be the most dominant calcifying algae, which blooms can even be seen from outer space. Calcifying algae create an exoskeleton
An exoskeleton (from Greek ''éxō'' "outer" and ''skeletós'' "skeleton") is an external skeleton that supports and protects an animal's body, in contrast to an internal skeleton (endoskeleton) in for example, a human. In usage, some of the ...
from calcium carbonate platelets (coccolith
Coccoliths are individual plates or scales of calcium carbonate formed by coccolithophores (single-celled phytoplankton such as ''Emiliania huxleyi'') and cover the cell surface arranged in the form of a spherical shell, called a ''coccosphere''. ...
s), providing ballast
Ballast is material that is used to provide stability to a vehicle or structure. Ballast, other than cargo, may be placed in a vehicle, often a ship or the gondola of a balloon or airship, to provide stability. A compartment within a boat, ship, ...
which enhances the organic and inorganic carbon flux to the deep sea. Organic carbon is formed by means of photosynthesis, where CO2 is fixed and converted into organic molecules, causing removal of CO2 from the seawater. Counterintuitively, the production of coccoliths leads to the release of CO2 in the seawater, due to removal of carbonate from the seawater, which reduces the alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of ...
and causes acidification
Acidification may refer to:
* Ocean acidification, decrease in the pH of the Earth's oceans
* Freshwater acidification, atmospheric depositions and soil leaching of SOx and NOx
* Soil acidification, buildup of hydrogen cations, which reduces the ...
. Therefore, the ratio between particulate inorganic carbon (PIC) and particulate organic carbon
Particulate organic matter (POM) is a fraction of total organic matter operationally defined as that which does not pass through a filter pore size that typically ranges in size from 0.053 to 2 millimeters.
Particulate organic carbon (POC) is ...
(POC) is an important measure for the net release or uptake of CO2. In short, the PIC:POC ratio is a key characteristic required to understand and predict the impact of climate change on the global ocean carbon cycle
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many inter ...
. Creative Commons Attribution 4.0 International License
Calcium particle morphologies

Coccolithus pelagicus
''Coccolithus'' is a genus of unicellular haptophytes.
Species
The species in this genus include:
*''Coccolithus oceanicus''
*''Coccolithus pelagicus''
*''Coccolithus pliopelagicus''
References
External links Imagesof ''Coccolithus'' a ...
''
File:Foram-globigerina hg.jpg, foraminiferan
Foraminifera (; Latin for "hole bearers"; informally called "forams") are single-celled organisms, members of a phylum or class of amoeboid protists characterized by streaming granular ectoplasm for catching food and other uses; and commonly an ...

See also
*carbonate compensation depth
Carbonate compensation depth (CCD) is the depth in the oceans below which the rate of supply of calcite ( calcium carbonate) lags behind the rate of solvation, such that no calcite is preserved. Shells of animals therefore dissolve and carbonate ...
* aragonite compensation depth
* lysocline
The lysocline is the depth in the ocean dependent upon the carbonate compensation depth (CCD), usually around 3.5 km, below which the rate of dissolution of calcite increases dramatically because of a pressure effect. While the lysocline is the u ...
* calcareous ooze
Calcareous () is an adjective meaning "mostly or partly composed of calcium carbonate", in other words, containing lime or being chalky. The term is used in a wide variety of scientific disciplines.
In zoology
''Calcareous'' is used as an adje ...
* Carbonate pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments.Sigman DM & GH ...
* Marine biogenic calcification
Marine biogenic calcification is the process by which marine organisms such as oysters and clams form calcium carbonate. Seawater is full of dissolved compounds, ions and nutrients that organisms can use for energy and, in the case of calcificatio ...
* snowline: the depth at which carbonate disappear from sediments under steady-state conditions
References
Sources
* * * * * * * * {{Cite journal , last1=Wilkinson , first1=B.H. , last2=Owen , first2=R.M. , last3=Carroll , first3=A.R. , year = 1985 , title = Submarine hydrothermal weathering, global eustacy, and carbonate polymorphism in Phanerozoic marine oolites , journal = Journal of Sedimentary Petrology , volume = 55 , pages = 171–183 , doi=10.1306/212f8657-2b24-11d7-8648000102c1865d Chemical oceanography Environmental chemistry Soil