|
Dissolved Inorganic Carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, , respectively). Dissolved inorganic carbon (DIC) includes three major aqueous species, CO2, ,, and to a lesser extent their complexes in solution with metal ions. Marine ecosystems Solubility pump Aqueous carbon dioxide reacts with water to form carbonic acid which is very unstable and will dissociate rapidly into hydronium and bicarbonate. Therefore, in seawater, dissolved inorganic carbon is commonly referred to as the collection of bicarbonate, carbonate ions, and dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annual DOC And DIC Fluxes In The Tanguro Ranch Watershed , in biology
{{disambiguation ...
Annual may refer to: *Annual publication, periodical publications appearing regularly once per year **Yearbook **Literary annual *Annual plant *Annual report *Annual giving *Annual, Morocco, a settlement in northeastern Morocco *Annuals (band), a musical group See also * Annual Review (other) * Circannual cycle A circannual cycle is a biological process that occurs in living creatures over the period of approximately one year. This cycle was first discovered by Ebo Gwinner and Canadian biologist Ted Pengelley. It is classified as an Infradian rhythm, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spatial Distribution Of Ocean Surface Dissolved Inorganic Carbon
Spatial may refer to: *Dimension *Space *Three-dimensional space Three-dimensional space (also: 3D space, 3-space or, rarely, tri-dimensional space) is a geometric setting in which three values (called ''parameters'') are required to determine the position (geometry), position of an element (i.e., Point (m ... See also * * {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Organic Carbon
Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins. A typical analysis for total carbon (TC) measures both the total organic carbon (TOC) present and the complementing total inorganic carbon (TIC), the latter representing the amount of non-organic carbon, like carbon in carbonate minerals. Subtracting the inorganic carbon from the total carbon yields TOC. Another common variant of TOC analysis involves removing the TIC portion first and then measuring the leftover carbon. This method involves purging an acidified sample with carbon-free air or nitrogen prior to mea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
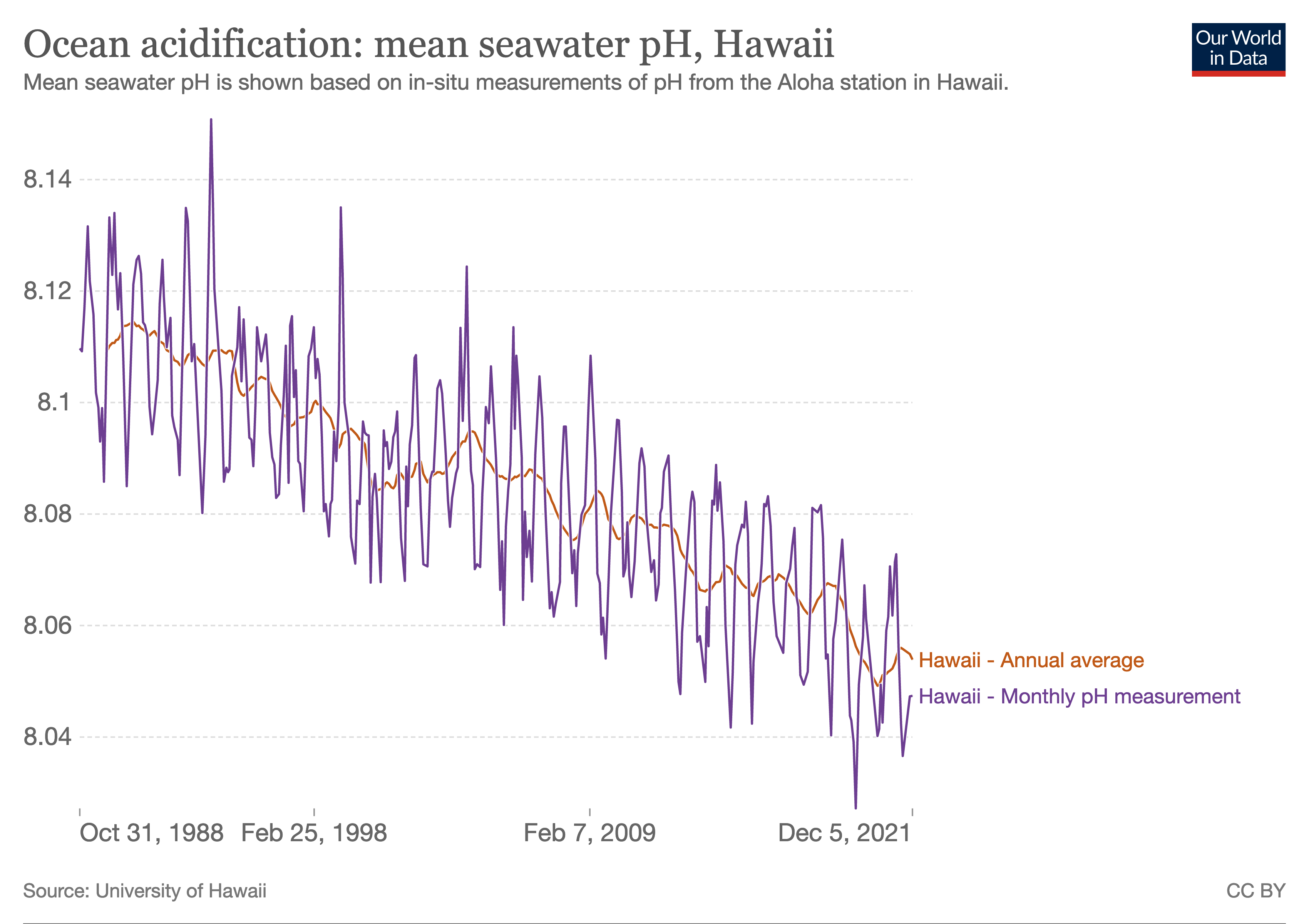
Ocean Acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxide emissions from human activities which have led to atmospheric carbon dioxide (CO2) levels of more than 410 ppm (in 2020). The oceans absorb CO2 from the atmosphere. This leads to the formation of carbonic acid (H2CO3) which dissociates into a bicarbonate ion () and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the pH of the ocean, therefore increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). A decrease in pH corresponds to a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. Marine calcifying organisms, like mollusks, oysters and corals, are particularly affected by this as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas. Fugacities are determined experimentally or estimated from various models such as a Van der Waals gas that are closer to reality than an ideal gas. The real gas pressure and fugacity are related through the dimensionless fugacity coefficient . \varphi = \frac For an ideal gas, fugacity and pressure are equal and so . Taken at the same temperature and pressure, the difference between the molar Gibbs free energies of a real gas and the corresponding ideal gas is equal to . The fugacity is closely related to the thermodynamic activity. For a gas, the activity is simply the fugacity divided by a reference pressure to give a dimensionless quantity. This reference press ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissolved Organic Carbon
Dissolved organic carbon (DOC) is the fraction of organic carbon operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometers. The fraction remaining on the filter is called particulate organic carbon (POC). Dissolved organic matter (DOM) is a closely related term often used interchangeably with DOC. While DOC refers specifically to the mass of carbon in the dissolved organic material, DOM refers to the total mass of the dissolved organic matter. So DOM also includes the mass of other elements present in the organic material, such as nitrogen, oxygen and hydrogen. DOC is a component of DOM and there is typically about twice as much DOM as DOC. Many statements that can be made about DOC apply equally to DOM, and ''vice versa''. DOC is abundant in marine and freshwater systems and is one of the greatest cycled reservoirs of organic matter on Earth, accounting for the same amount of carbon as in the atmosphere and up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bjerrum Plot
A Bjerrum plot (named after Niels Bjerrum; sometimes also known as a Sillén diagram or a Hägg diagram) is a graph of the concentrations of the different species of a polyprotic acid in a solution, as a function of pH, when the solution is at equilibrium. Due to the many orders of magnitude spanned by the concentrations, they are commonly plotted on a logarithmic scale. Sometimes the ratios of the concentrations are plotted rather than the actual concentrations. Occasionally H+ and OH− are also plotted. Most often, the carbonate system is plotted, where the polyprotic acid is carbonic acid (a diprotic acid), and the different species are dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate. In acidic conditions, the dominant form is ; in basic (alkaline) conditions, the dominant form is ; and in between, the dominant form is . At every pH, the concentration of carbonic acid is assumed to be negligible compared to the concentration of dissolved , and so is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. Although alkalinity is primarily a term used by oceanographers, it is also used by hydrologists to describe temporary hardness. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from rainfall or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particulate Inorganic Carbon
Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In Operational definition, operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon. Most PIC is calcium carbonate, CaCO3, particularly in the form of calcite, but also in the form of aragonite. Calcium carbonate makes up the shells of many marine organisms. It also forms during whiting events and is excreted by marine fish during osmoregulation. Overview Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
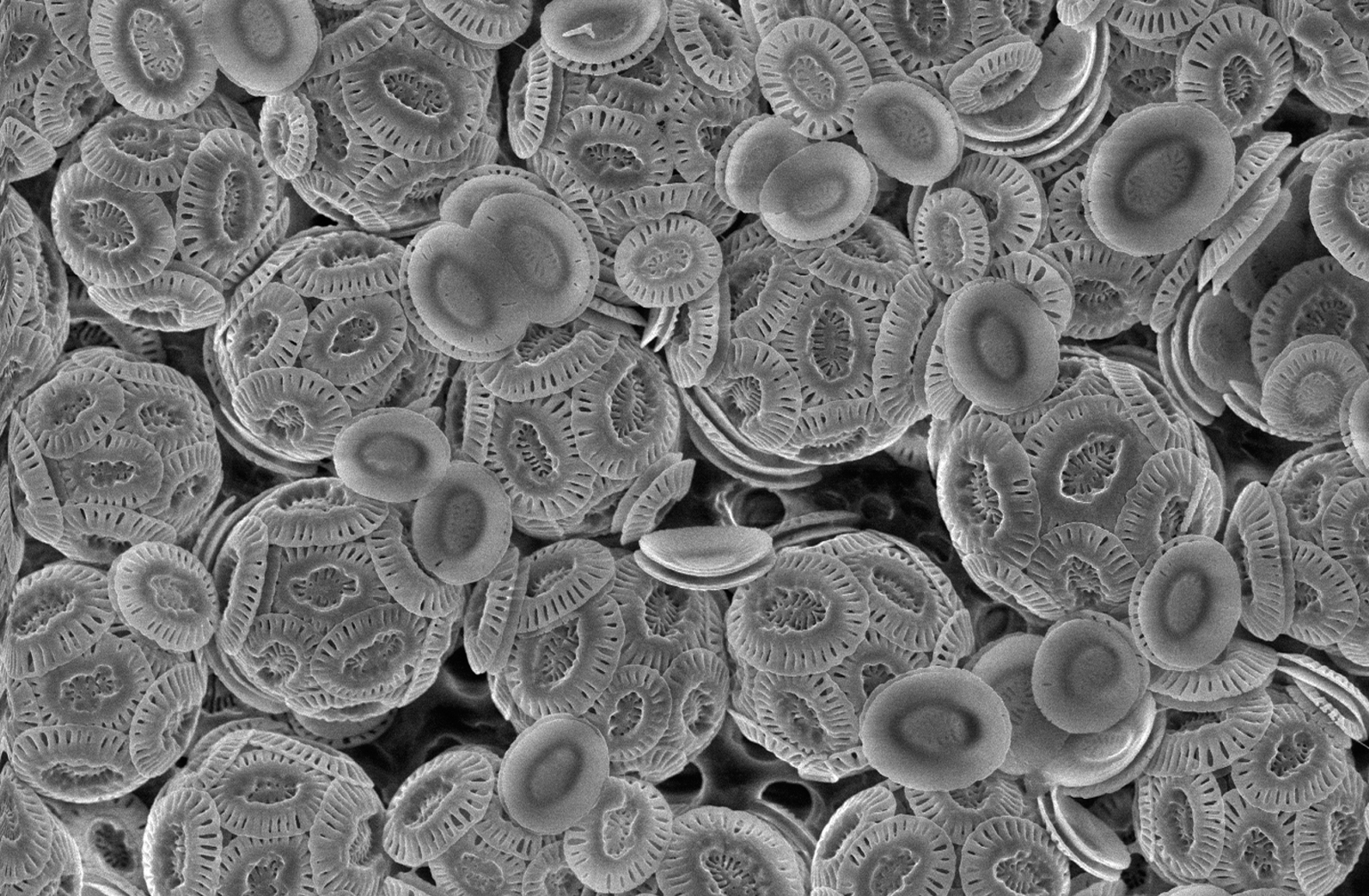
Coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the kingdom Protista, according to Robert Whittaker's Five kingdom classification, or clade Hacrobia, according to a newer biological classification system. Within the Hacrobia, the coccolithophores are in the phylum or division Haptophyta, class Prymnesiophyceae (or Coccolithophyceae). Coccolithophores are almost exclusively marine, are photosynthetic, and exist in large numbers throughout the sunlight zone of the ocean. Coccolithophores are the most productive calcifying organisms on the planet, covering themselves with a calcium carbonate shell called a ''coccosphere''. However, the reasons they calcify remains elusive. One key function may be that the coccosphere offers protection against microzooplankton predation, which is one of the ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going from hand production methods to machines, new chemical manufacturing and iron production processes, the increasing use of steam power and water power, the development of machine tools and the rise of the mechanized factory system. Output greatly increased, and a result was an unprecedented rise in population and in the rate of population growth. Textiles were the dominant industry of the Industrial Revolution in terms of employment, value of output and capital invested. The textile industry was also the first to use modern production methods. The Industrial Revolution began in Great Britain, and many of the technological and architectural innovations were of British origin. By the mid-18th century, Britain was the world's leadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_map_2014.png)