Macrophage on:
[Wikipedia]
[Google]
[Amazon]
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the
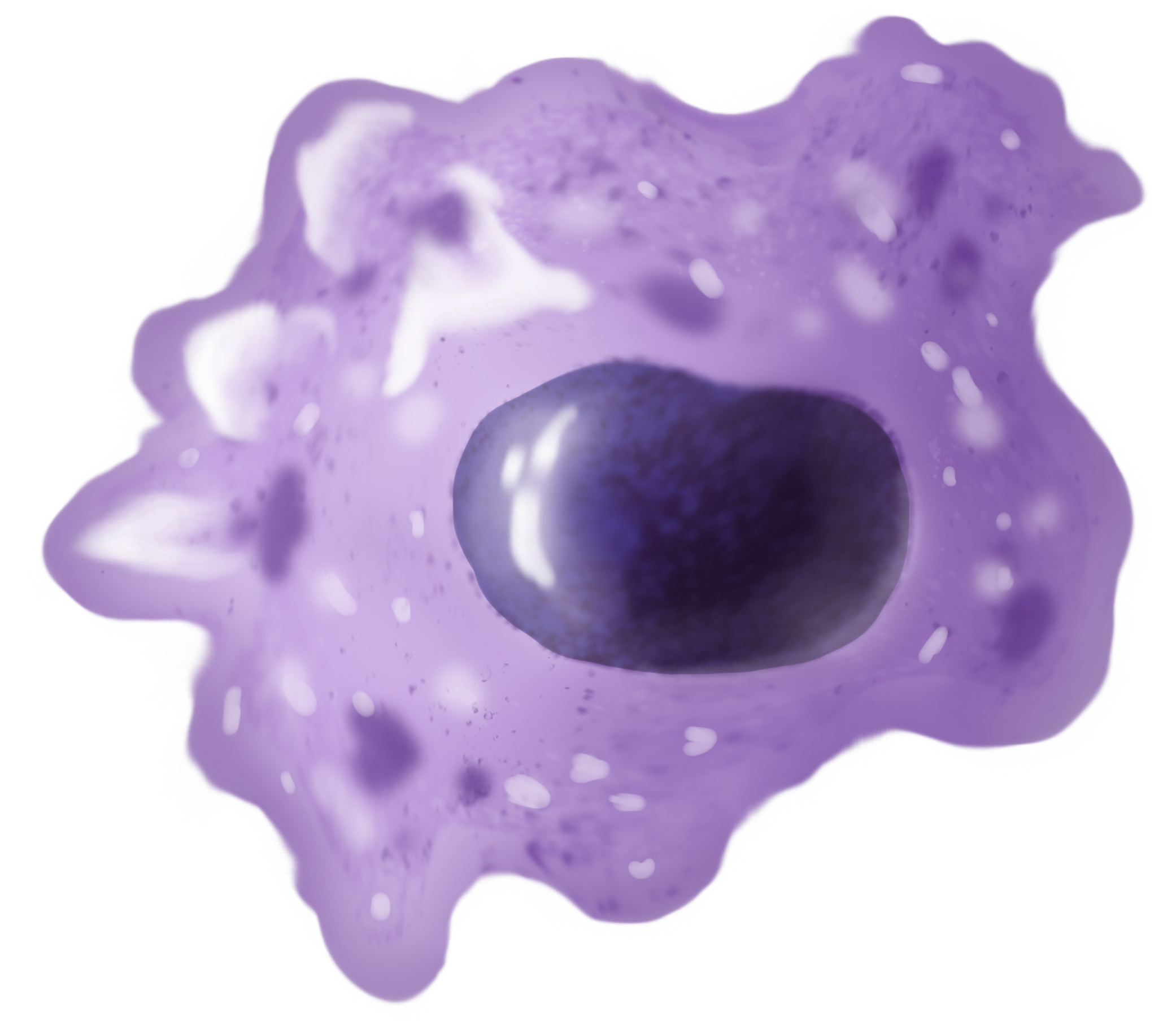 A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the
A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the

 Macrophages are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and other debris. Along with
Macrophages are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and other debris. Along with
Wound healing: Chronic wounds
Emedicine.com. Accessed 20 January 2008. They replace
Wound Healing, Growth Factors
Emedicine.com. Accessed 20 January 2008. Macrophages may also restrain the contraction phase. Macrophages are stimulated by the low
 Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as
Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as
File:S4-J774 Cells with Conidia in Liquid Media.ogg, An active J774 macrophage is seen taking up four conidia in a co-operative manner. The J774 cells were treated with 5 ng/ml
The role of macrophages in HIV pathogenesis
Macrophages News
Macrophages News provided by insciences organisation
www.macrophages.com
The Macrophage Community Website {{Authority control Phagocytes Cell biology Immune system Human cells Articles containing video clips Connective tissue cells Lymphatic system
immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinte ...
that engulfs and digests pathogens, such as cancer cell
Cancer cells are cells that divide continually, forming solid tumors or flooding the blood with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells, and these d ...
s, microbe
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. The process is called phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is ...
, which acts to defend the host against infection and injury.
These large phagocyte
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ...
s are found in essentially all tissues, where they patrol for potential pathogen
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
s by amoeboid movement
Amoeboid movement is the most typical mode of locomotion in adherent eukaryotic cells. It is a crawling-like type of movement accomplished by protrusion of cytoplasm of the cell involving the formation of pseudopodia ("false-feet") and posterior ...
. They take various forms (with various names) throughout the body (e.g., histiocyte
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system (also known as the reticuloendothelial system or lymphoreticular system). The mononuclear phagocytic system is part of the organism's immune system. The histiocyt ...
s, Kupffer cell
Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Ku ...
s, alveolar macrophage
An alveolar macrophage, pulmonary macrophage, (or dust cell) is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.
Activity of the alveolar macropha ...
s, microglia, and others), but all are part of the mononuclear phagocyte system
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS) also known as the reticuloendothelial system or macrophage system is a part of the immune system that consists of the phagocytic cells located in reticular co ...
. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity
The innate, or nonspecific, immune system is one of the two main immunity strategies (the other being the adaptive immune system) in vertebrates. The innate immune system is an older evolutionary defense strategy, relatively speaking, and is the ...
) and also help initiate specific defense mechanisms (adaptive immunity
The adaptive immune system, also known as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminate pathogens or prevent their growth. The acquired immune system ...
) by recruiting other immune cells such as lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
s. For example, they are important as antigen presenters to T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
s. In humans, dysfunctional macrophages cause severe diseases such as chronic granulomatous disease
Chronic granulomatous disease (CGD), also known as Bridges–Good syndrome, chronic granulomatous disorder, and Quie syndrome, is a diverse group of hereditary diseases in which certain cells of the immune system have difficulty forming the reacti ...
that result in frequent infections.
Beyond increasing inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
and stimulating the immune system, macrophages also play an important anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs remedy pain by reducing inflammation as o ...
role and can decrease immune reactions through the release of cytokines. Macrophages that encourage inflammation are called M1 macrophages, whereas those that decrease inflammation and encourage tissue repair are called M2 macrophages. This difference is reflected in their metabolism; M1 macrophages have the unique ability to metabolize arginine to the "killer" molecule nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its che ...
, whereas M2 macrophages have the unique ability to metabolize arginine to the "repair" molecule ornithine
Ornithine is a non-proteinogenic amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.
Role in urea cycle
L-Ornithine is one of the produ ...
. However, this dichotomy has been recently questioned as further complexity has been discovered.
Human macrophages are about in diameter and are produced by the differentiation of monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s in tissues. They can be identified using flow cytometry or immunohistochemical staining
Immunohistochemistry (IHC) is the most common application of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to ant ...
by their specific expression of proteins such as CD14
CD14 (cluster of differentiation 14) is a human protein made mostly by macrophages as part of the innate immune system. It helps to detect bacteria in the body by binding lipopolysaccharide (LPS), a pathogen-associated molecular pattern (PAMP).
...
, CD40
Cluster of differentiation 40, CD40 is a costimulatory protein found on antigen-presenting cells and is required for their activation. The binding of CD154 ( CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of d ...
, CD11b
Integrin alpha M (ITGAM) is one protein subunit that forms heterodimeric integrin alpha-M beta-2 (αMβ2) molecule, also known as ''macrophage-1 antigen'' (Mac-1) or '' complement receptor 3'' (CR3). ITGAM is also known as CR3A, and cluster of dif ...
, CD64, F4/80 (mice)/EMR1
EGF-like module-containing mucin-like hormone receptor-like 1 also known as F4/80 is a protein encoded by the ''ADGRE1'' gene. EMR1 is a member of the adhesion GPCR family.
Adhesion GPCRs are characterized by an extended extracellular region oft ...
(human), lysozyme
Lysozyme (EC 3.2.1.17, muramidase, ''N''-acetylmuramide glycanhydrolase; systematic name peptidoglycan ''N''-acetylmuramoylhydrolase) is an antimicrobial enzyme produced by animals that forms part of the innate immune system. It is a glycoside ...
M, MAC-1/MAC-3 and CD68
CD68 ( Cluster of Differentiation 68) is a protein highly expressed by cells in the monocyte lineage (e.g., monocytic phagocytes, osteoclasts), by circulating macrophages, and by tissue macrophages (e.g., Kupffer cells, microglia).
Structure a ...
.
Macrophages were first discovered and named by Élie Metchnikoff
Ilya Ilyich Mechnikov (russian: Илья Ильич Мечников; – 15 July 1916), also spelled Élie Metchnikoff, was a Russian zoologist best known for his pioneering research in immunology. Belkin, a Russian science historian, explain ...
, a Russian zoologist, in 1884.
Structure
Types
 A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the
A majority of macrophages are stationed at strategic points where microbial invasion or accumulation of foreign particles is likely to occur. These cells together as a group are known as the mononuclear phagocyte system
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS) also known as the reticuloendothelial system or macrophage system is a part of the immune system that consists of the phagocytic cells located in reticular co ...
and were previously known as the reticuloendothelial system. Each type of macrophage, determined by its location, has a specific name:
Investigations concerning Kupffer cells are hampered because in humans, Kupffer cells are only accessible for immunohistochemical analysis from biopsies or autopsies. From rats and mice, they are difficult to isolate, and after purification, only approximately 5 million cells can be obtained from one mouse.
Macrophages can express paracrine Paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse over ...
functions within organs that are specific to the function of that organ. In the testis
A testicle or testis (plural testes) is the male reproductive gland or gonad in all bilaterians, including humans. It is homologous to the female ovary. The functions of the testes are to produce both sperm and androgens, primarily testostero ...
, for example, macrophages have been shown to be able to interact with Leydig cells
Leydig cells, also known as interstitial cells of the testes and interstitial cells of Leydig, are found adjacent to the seminiferous tubules in the testicle and produce testosterone in the presence of luteinizing hormone (LH). They are polyhedra ...
by secreting 25-hydroxycholesterol, an oxysterol
An oxysterol is a derivative of cholesterol obtained by oxidation involving enzymes and / or pro-oxidants. Such compounds play important roles in various biological processes such as cholesterol homeostasis, lipid metabolism (sphingolipids, fatty ...
that can be converted to testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristi ...
by neighbouring Leydig cells. Also, testicular macrophages may participate in creating an immune privileged environment in the testis, and in mediating infertility during inflammation of the testis.
Cardiac resident macrophages participate in electrical conduction via gap junction communication with cardiac myocyte
A muscle cell is also known as a myocyte when referring to either a cardiac muscle cell (cardiomyocyte), or a smooth muscle cell as these are both small cells. A skeletal muscle cell is long and threadlike with many nuclei and is called a mus ...
s.
Macrophages can be classified on basis of the fundamental function and activation. According to this grouping there are classically-activated (M1) macrophages, wound-healing macrophages (also known as alternatively-activated (M2) macrophages), and regulatory macrophages Regulatory macrophages (Mregs) represent a subset of anti-inflammatory macrophages. In general, macrophages are a very dynamic and plastic cell type and can be divided into two main groups: classically activated macrophages (M1) and alternatively ac ...
(Mregs).
Development
Macrophages that reside in adult healthy tissues either derive from circulating monocytes or are established before birth and then maintained during adult life independently of monocytes. By contrast, most of the macrophages that accumulate at diseased sites typically derive from circulating monocytes.Leukocyte extravasation
Leukocyte extravasation (also commonly known as leukocyte adhesion cascade or diapedesis – the passage of cells through the intact vessel wall) is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or ...
describes monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
entry into damaged tissue through the endothelium
The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vesse ...
of blood vessels as they become macrophages. Monocytes are attracted to a damaged site by chemical substances through chemotaxis, triggered by a range of stimuli including damaged cells, pathogens and cytokines released by macrophages already at the site. At some sites such as the testis, macrophages have been shown to populate the organ through proliferation. Unlike short-lived neutrophils, macrophages survive longer in the body, up to several months.
Function
Phagocytosis
Macrophages are professional phagocytes and are highly specialized in removal of dying or dead cells and cellular debris. This role is important in chronic inflammation, as the early stages of inflammation are dominated by neutrophils, which are ingested by macrophages if they come of age (seeCD31
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is a protein that in humans is encoded by the ''PECAM1'' gene found on chromosome17q23.3. PECAM-1 plays a key role in removing aged neutrop ...
for a description of this process).
The neutrophils are at first attracted to a site, where they perform their function and die, before they or their neutrophil extracellular traps
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens. Neutrophils are the immune system's first line of defense against infection and have conventionally be ...
are phagocytized by the macrophages. When at the site, the first wave of neutrophils, after the process of aging and after the first 48 hours, stimulate the appearance of the macrophages whereby these macrophages will then ingest the aged neutrophils.
The removal of dying cells is, to a greater extent, handled by ''fixed macrophages'', which will stay at strategic locations such as the lungs, liver, neural tissue, bone, spleen and connective tissue, ingesting foreign materials such as pathogens and recruiting additional macrophages if needed.
When a macrophage ingests a pathogen, the pathogen becomes trapped in a phagosome
In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).
A phagosome is formed by the fusion of the cell ...
, which then fuses with a lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane pr ...
. Within the phagolysosome, enzymes and toxic peroxides digest the pathogen. However, some bacteria, such as ''Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb) is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, ''M. tuberculosis'' has an unusual, waxy coating on its c ...
'', have become resistant to these methods of digestion. Typhoidal ''Salmonellae'' induce their own phagocytosis by host macrophages in vivo, and inhibit digestion by lysosomal action, thereby using macrophages for their own replication and causing macrophage apoptosis. Macrophages can digest more than 100 bacteria before they finally die due to their own digestive compounds.
Role in adaptive immunity
 Macrophages are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and other debris. Along with
Macrophages are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and other debris. Along with dendritic cells
Dendritic cells (DCs) are antigen-presenting cells (also known as ''accessory cells'') of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. The ...
, they are foremost among the cells that present antigens, a crucial role in initiating an immune response. As secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the development of inflammation; they produce a wide array of powerful chemical substances (monokine A monokine is a type of cytokine produced primarily by monocytes and macrophages.
Some monokines are:
* interleukin 1
* tumor necrosis factor-alpha
* alpha and beta interferon
* colony stimulating factors
Functions
Monokines released from macroph ...
s) including enzymes, complement proteins, and regulatory factors such as interleukin-1
The Interleukin-1 family (IL-1 family) is a group of 11 cytokines that plays a central role in the regulation of immune and inflammatory responses to infections or sterile insults.
Discovery
Discovery of these cytokines began with studies on t ...
. At the same time, they carry receptors for lymphokine Lymphokines are a subset of cytokines that are produced by a type of immune cell known as a lymphocyte. They are protein mediators typically produced by T cells to direct the immune system response by signaling between its cells. Lymphokines have ...
s that allow them to be "activated" into single-minded pursuit of microbes and tumour cells.
After digesting a pathogen, a macrophage will present the antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune respons ...
(a molecule, most often a protein found on the surface of the pathogen and used by the immune system for identification) of the pathogen to the corresponding helper T cell
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are consider ...
. The presentation is done by integrating it into the cell membrane and displaying it attached to an MHC class II molecule (MHCII), indicating to other white blood cells that the macrophage is not a pathogen, despite having antigens on its surface.
Eventually, the antigen presentation results in the production of antibodies that attach to the antigens of pathogens, making them easier for macrophages to adhere to with their cell membrane and phagocytose. In some cases, pathogens are very resistant to adhesion by the macrophages.
The antigen presentation on the surface of infected macrophages (in the context of MHC class II) in a lymph node stimulates TH1 (type 1 helper T cells) to proliferate (mainly due to IL-12 secretion from the macrophage). When a B-cell in the lymph node recognizes the same unprocessed surface antigen on the bacterium with its surface bound antibody, the antigen is endocytosed and processed. The processed antigen is then presented in MHCII on the surface of the B-cell. T cells that express the T cell receptor which recognizes the antigen-MHCII complex (with co-stimulatory factors- CD40
Cluster of differentiation 40, CD40 is a costimulatory protein found on antigen-presenting cells and is required for their activation. The binding of CD154 ( CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of d ...
and CD40L
CD154, also called CD40 ligand or CD40L, is a protein that is primarily expressed on activated T cells and is a member of the TNF superfamily of molecules. It binds to CD40 on antigen-presenting cells (APC), which leads to many effects dependin ...
) cause the B-cell to produce antibodies that help opsonisation of the antigen so that the bacteria can be better cleared by phagocytes
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek '' ...
.
Macrophages provide yet another line of defense against tumor cells and somatic cells infected with fungus
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from th ...
or parasite
Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson has ...
s. Once a T cell has recognized its particular antigen on the surface of an aberrant cell, the T cell becomes an activated effector cell, producing chemical mediators known as lymphokines that stimulate macrophages into a more aggressive form.
Macrophage subtypes
There are several activated forms of macrophages. In spite of a spectrum of ways to activate macrophages, there are two main groups designated M1 and M2. M1 macrophages: as mentioned earlier (previously referred to as classically activated macrophages), M1 "killer" macrophages are activated by LPS andIFN-gamma
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock ...
, and secrete high levels of IL-12 and low levels of IL-10. M1 macrophages have pro-inflammatory, bactericidal, and phagocytic functions. In contrast, the M2 "repair" designation (also referred to as alternatively activated macrophages) broadly refers to macrophages that function in constructive processes like wound healing and tissue repair, and those that turn off damaging immune system activation by producing anti-inflammatory cytokines like IL-10. M2 is the phenotype of resident tissue macrophages, and can be further elevated by IL-4. M2 macrophages produce high levels of IL-10, TGF-beta
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other ...
and low levels of IL-12. Tumor-associated macrophages are mainly of the M2 phenotype, and seem to actively promote tumor growth.
Macrophages exist in a variety of phenotypes which are determined by the role they play in wound maturation. Phenotypes can be predominantly separated into two major categories; M1 and M2. M1 macrophages are the dominating phenotype observed in the early stages of inflammation and are activated by four key mediators: interferon-γ (IFN-γ), tumor necrosis factor (TNF), and damage associated molecular patterns (DAMPs). These mediator molecules create a pro-inflammatory response that in return produce pro-inflammatory cytokines like Interleukin-6 and TNF. Unlike M1 macrophages, M2 macrophages secrete an anti-inflammatory response via the addition of Interleukin-4 or Interleukin-13. They also play a role in wound healing and are needed for revascularization and reepithelialization. M2 macrophages are divided into four major types based on their roles: M2a, M2b, M2c, and M2d. How M2 phenotypes are determined is still up for discussion but studies have shown that their environment allows them to adjust to whichever phenotype is most appropriate to efficiently heal the wound.
M2 macrophages are needed for vascular stability. They produce vascular endothelial growth factor-A and TGF-β1. There is a phenotype shift from M1 to M2 macrophages in acute wounds, however this shift is impaired for chronic wounds. This dysregulation results in insufficient M2 macrophages and its corresponding growth factors that aid in wound repair. With a lack of these growth factors/anti-inflammatory cytokines and an overabundance of pro-inflammatory cytokines from M1 macrophages chronic wounds are unable to heal in a timely manner. Normally, after neutrophils eat debris/pathogens they perform apoptosis and are removed. At this point, inflammation is not needed and M1 undergoes a switch to M2 (anti-inflammatory). However, dysregulation occurs as the M1 macrophages are unable/do not phagocytose neutrophils that have undergone apoptosis leading to increased macrophage migration and inflammation.
Both M1 and M2 macrophages play a role in promotion of atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheromatous plaque. At onset there are usually no s ...
. M1 macrophages promote atherosclerosis by inflammation. M2 macrophages can remove cholesterol from blood vessels, but when the cholesterol is oxidized, the M2 macrophages become apoptotic
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes incl ...
foam cells
Foam cells, also called lipid-laden macrophages, are a type of cell that contain cholesterol. These can form a plaque that can lead to atherosclerosis and trigger heart attacks and stroke.
Foam cells are fat-laden cells with a M2 macrophage-lik ...
contributing to the atheromatous plaque
An atheroma, or atheromatous plaque, is an abnormal and reversible accumulation of material in the inner layer of an arterial wall.
The material consists of mostly macrophage cells, or debris, containing lipids, calcium and a variable amount o ...
of atherosclerosis.
Role in muscle regeneration
The first step to understanding the importance of macrophages in muscle repair, growth, and regeneration is that there are two "waves" of macrophages with the onset of damageable muscle use – subpopulations that do and do not directly have an influence on repairing muscle. The initial wave is a phagocytic population that comes along during periods of increased muscle use that are sufficient to cause muscle membrane lysis and membrane inflammation, which can enter and degrade the contents of injured muscle fibers. These early-invading, phagocytic macrophages reach their highest concentration about 24 hours following the onset of some form of muscle cell injury or reloading. Their concentration rapidly declines after 48 hours. The second group is the non-phagocytic types that are distributed near regenerative fibers. These peak between two and four days and remain elevated for several days during while muscle tissue is rebuilding. The first subpopulation has no direct benefit to repairing muscle, while the second non-phagocytic group does. It is thought that macrophages release soluble substances that influence the proliferation, differentiation, growth, repair, and regeneration of muscle, but at this time the factor that is produced to mediate these effects is unknown. It is known that macrophages' involvement in promoting tissue repair is not muscle specific; they accumulate in numerous tissues during the healing process phase following injury.Role in wound healing
Macrophages are essential forwound healing
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
In undamaged skin, the epidermis (surface, epithelial layer) and dermis (deeper, connective layer) form a protective barrier again ...
.de la Torre J., Sholar A. (2006)Wound healing: Chronic wounds
Emedicine.com. Accessed 20 January 2008. They replace
polymorphonuclear neutrophil
Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying in ...
s as the predominant cells in the wound by day two after injury. Attracted to the wound site by growth factors released by platelets and other cells, monocyte
Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and conventional dendritic cells. As a part of the vertebrate innate immune system monocytes also ...
s from the bloodstream enter the area through blood vessel walls. Numbers of monocytes in the wound peak one to one and a half days after the injury occurs. Once they are in the wound site, monocytes mature into macrophages. The spleen
The spleen is an organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter. The word spleen comes .
contains half the body's monocytes in reserve ready to be deployed to injured tissue.
The macrophage's main role is to phagocytize bacteria and damaged tissue, and they also debride
Debridement is the medical removal of dead, damaged, or infected tissue to improve the healing potential of the remaining healthy tissue. Removal may be surgical, mechanical, chemical, autolytic (self-digestion), and by maggot therapy.
In po ...
damaged tissue by releasing proteases. Macrophages also secrete a number of factors such as growth factors and other cytokines, especially during the third and fourth post-wound days. These factors attract cells involved in the proliferation stage of healing to the area.Rosenberg L., de la Torre J. (2006)Wound Healing, Growth Factors
Emedicine.com. Accessed 20 January 2008. Macrophages may also restrain the contraction phase. Macrophages are stimulated by the low
oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
content of their surroundings to produce factors that induce and speed angiogenesis and they also stimulate cells that re-epithelialize the wound, create granulation tissue, and lay down a new extracellular matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide s ...
. By secreting these factors, macrophages contribute to pushing the wound healing process into the next phase.
Role in limb regeneration
Scientists have elucidated that as well as eating up material debris, macrophages are involved in the typicallimb regeneration
In biology, regeneration is the process of renewal, restoration, and tissue growth that makes genomes, cells, organisms, and ecosystems resilient to natural fluctuations or events that cause disturbance or damage. Every species is capable of reg ...
in the salamander. They found that removing the macrophages from a salamander
Salamanders are a group of amphibians typically characterized by their lizard-like appearance, with slender bodies, blunt snouts, short limbs projecting at right angles to the body, and the presence of a tail in both larvae and adults. All t ...
resulted in failure of limb regeneration and a scarring response.
Role in iron homeostasis
As described above, macrophages play a key role in removing dying or dead cells and cellular debris.Erythrocytes
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
have a lifespan on average of 120 days and so are constantly being destroyed by macrophages in the spleen and liver. Macrophages will also engulf macromolecules, and so play a key role in the pharmacokinetics of parenteral iron
Iron supplements, also known as iron salts and iron pills, are a number of iron formulations used to treat and prevent iron deficiency including iron deficiency anemia. For prevention they are only recommended in those with poor absorption, ...
s.
The iron that is released from the haemoglobin is either stored internally in ferritin
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary ' ...
or is released into the circulation via ferroportin
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the ''SLC40A1'' gene, and is part of the Ferroportin (Fpn) FamilyTC# 2.A.100. Ferroportin i ...
. In cases where systemic iron levels are raised, or where inflammation is present, raised levels of hepcidin
Hepcidin is a protein that in humans is encoded by the ''HAMP'' gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
During conditions in which the hepcidin level is abnormally high, such as inflammation, se ...
act on macrophage ferroportin channels, leading to iron remaining within the macrophages.
Role in pigment retainment
 Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as
Melanophages are a subset of tissue-resident macrophages able to absorb pigment, either native to the organism or exogenous (such as tattoo
A tattoo is a form of body modification made by inserting tattoo ink, dyes, and/or pigments, either indelible or temporary, into the dermis layer of the skin to form a design. Tattoo artists create these designs using several Process of tatt ...
s), from extracellular space. In contrast to dendritic juncional melanocytes, which synthesize melanosomes and contain various stages of their development, the melanophages only accumulate phagocytosed
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is c ...
melanin in lysosome-like phagosomes. This occurs repeatedly as the pigment from dead dermal macrophages is phagocytosed by their successors, preserving the tattoo in the same place.
Role in tissue homeostasis
Every tissue harbors its own specialized population of resident macrophages, which entertain reciprocal interconnections with the stroma and functional tissue. These resident macrophages are sessile (non-migratory), provide essential growth factors to support the physiological function of the tissue (e.g. macrophage-neuronal crosstalk in the guts), and can actively protect the tissue from inflammatory damage.Nerve-associated macrophages
Nerve-associated macrophages or NAMs are those tissue-resident macrophages that are associated with nerves. Some of them are known to have an elongated morphology of up to 200μmClinical significance
Due to their role in phagocytosis, macrophages are involved in many diseases of the immune system. For example, they participate in the formation of granulomas, inflammatory lesions that may be caused by a large number of diseases. Some disorders, mostly rare, of ineffective phagocytosis and macrophage function have been described, for example.As a host for intracellular pathogens
In their role as a phagocytic immune cell macrophages are responsible for engulfing pathogens to destroy them. Some pathogens subvert this process and instead live inside the macrophage. This provides an environment in which the pathogen is hidden from the immune system and allows it to replicate. Diseases with this type of behaviour includetuberculosis
Tuberculosis (TB) is an infectious disease usually caused by '' Mycobacterium tuberculosis'' (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in ...
(caused by ''Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb) is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, ''M. tuberculosis'' has an unusual, waxy coating on its c ...
'') and leishmaniasis (caused by '' Leishmania'' species).
In order to minimize the possibility of becoming the host of an intracellular bacteria, macrophages have evolved defense mechanisms such as induction of nitric oxide and reactive oxygen intermediates, which are toxic to microbes. Macrophages have also evolved the ability to restrict the microbe's nutrient supply and induce autophagy.
Tuberculosis
Once engulfed by a macrophage, the causative agent of tuberculosis, ''Mycobacterium tuberculosis'', avoids cellular defenses and uses the cell to replicate. Recent evidence suggests that in response to the pulmonary infection of ''Mycobacterium tuberculosis'', the peripheral macrophages matures into M1 phenotype. Macrophage M1 phenotype is characterized by increased secretion of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) and increased glycolytic activities essential for clearance of infection.Leishmaniasis
Upon phagocytosis by a macrophage, the ''Leishmania'' parasite finds itself in a phagocytic vacuole. Under normal circumstances, this phagocytic vacuole would develop into a lysosome and its contents would be digested. ''Leishmania'' alter this process and avoid being destroyed; instead, they make a home inside the vacuole.Chikungunya
Infection of macrophages in joints is associated with local inflammation during and after the acute phase of ''Chikungunya
Chikungunya is an infection caused by the ''Chikungunya virus'' (CHIKV). Symptoms include fever and joint pains. These typically occur two to twelve days after exposure. Other symptoms may include headache, muscle pain, joint swelling, and a ra ...
'' (caused by CHIKV or Chikungunya virus).
Others
Adenovirus (most common cause of pink eye) can remain latent in a host macrophage, with continued viral shedding 6–18 months after initial infection. ''Brucella spp.'' can remain latent in a macrophage via inhibition ofphagosome
In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Professional phagocytes include macrophages, neutrophils, and dendritic cells (DCs).
A phagosome is formed by the fusion of the cell ...
–lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane pr ...
fusion; causes brucellosis
Brucellosis is a highly contagious zoonosis caused by ingestion of unpasteurized milk or undercooked meat from infected animals, or close contact with their secretions. It is also known as undulant fever, Malta fever, and Mediterranean fever.
The ...
(undulant fever).
'' Legionella pneumophila'', the causative agent of Legionnaires' disease, also establishes residence within macrophages.
Heart disease
Macrophages are the predominant cells involved in creating the progressive plaque lesions ofatherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheromatous plaque. At onset there are usually no s ...
.
Focal recruitment of macrophages occurs after the onset of acute myocardial infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may ...
. These macrophages function to remove debris, apoptotic cells and to prepare for tissue regeneration
In biology, regeneration is the process of renewal, restoration, and tissue growth that makes genomes, cells, organisms, and ecosystems resilient to natural fluctuations or events that cause disturbance or damage. Every species is capable of rege ...
. Macrophages protect against ischemia-induced ventricular tachycardia in hypokalemic mice.
HIV infection
Macrophages also play a role inhuman immunodeficiency virus
The human immunodeficiency viruses (HIV) are two species of ''Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immun ...
(HIV) infection. Like T cells
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell re ...
, macrophages can be infected with HIV, and even become a reservoir of ongoing virus replication throughout the body. HIV can enter the macrophage through binding of gp120 to CD4 and second membrane receptor, CCR5 (a chemokine receptor). Both circulating monocytes and macrophages serve as a reservoir for the virus. Macrophages are better able to resist infection by HIV-1 than CD4+ T cells, although susceptibility to HIV infection differs among macrophage subtypes.
Cancer
Macrophages can contribute to tumor growth and progression by promoting tumor cell proliferation and invasion, fostering tumor angiogenesis and suppressing antitumor immune cells. Attracted to oxygen-starved (hypoxic
Hypoxia means a lower than normal level of oxygen, and may refer to:
Reduced or insufficient oxygen
* Hypoxia (environmental), abnormally low oxygen content of the specific environment
* Hypoxia (medical), abnormally low level of oxygen in the t ...
) and necrotic tumor cells they promote chronic inflammation
Chronic systemic inflammation (SI) is the result of release of pro-inflammatory cytokines from immune-related cells and the chronic activation of the innate immune system. It can contribute to the development or progression of certain conditions ...
. Inflammatory compounds such as tumor necrosis factor
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homolog ...
(TNF)-alpha released by the macrophages activate the gene switch nuclear factor-kappa B. NF-κB then enters the nucleus of a tumor cell and turns on production of proteins that stop apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
and promote cell proliferation and inflammation. Moreover, macrophages serve as a source for many pro-angiogenic factors including vascular endothelial factor (VEGF), tumor necrosis factor-alpha
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homolog ...
(TNF-alpha), macrophage colony-stimulating factor
The colony stimulating factor 1 (CSF1), also known as macrophage colony-stimulating factor (M-CSF), is a secreted cytokine which causes hematopoietic stem cells to differentiate into macrophages or other related cell types. Eukaryotic cells also ...
(M-CSF/CSF1) and IL-1 and IL-6 contributing further to the tumor growth. Macrophages have been shown to infiltrate a number of tumors. Their number correlates with poor prognosis in certain cancers including cancers of breast, cervix, bladder, brain and prostate. Tumor-associated macrophages (TAMs) are thought to acquire an M2 phenotype, contributing to tumor growth and progression. Some tumors can also produce factors, including M-CSF/CSF1, MCP-1/CCL2 and Angiotensin II, that trigger the amplification and mobilization of macrophages in tumors. Research in various study models suggests that macrophages can sometimes acquire anti-tumor functions. For example, macrophages may have cytotoxic activity to kill tumor cells directly; also the co-operation of T-cells and macrophages is important to suppress tumors. This co-operation involves not only the direct contact of T-cell and macrophage, with antigen presentation, but also includes the secretion of adequate combinations of cytokines, which enhance T-cell antitumor activity. Recent study findings suggest that by forcing IFN-α expression in tumor-infiltrating macrophages, it is possible to blunt their innate protumoral activity and reprogram the tumor microenvironment toward more effective dendritic cell activation and immune effector cell cytotoxicity. Additionally, subcapsular sinus macrophages in tumor-draining lymph nodes can suppress cancer progression by containing the spread of tumor-derived materials.
Cancer therapy
Experimental studies indicate that macrophages can affect all therapeutic modalities, including surgery,chemotherapy
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs ( chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemothe ...
, radiotherapy, immunotherapy
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
and targeted therapy
Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment ( pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks ...
. Macrophages can influence treatment outcomes both positively and negatively. Macrophages can be protective in different ways: they can remove dead tumor cells (in a process called phagocytosis
Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is ...
) following treatments that kill these cells; they can serve as drug depots for some anticancer drugs; they can also be activated by some therapies to promote antitumor immunity. Macrophages can also be deleterious in several ways: for example they can suppress various chemotherapies, radiotherapies and immunotherapies. Because macrophages can regulate tumor progression, therapeutic strategies to reduce the number of these cells, or to manipulate their phenotypes, are currently being tested in cancer patients. However, macrophages are also involved in antibody mediated cytotoxicity (ADCC) and this mechanism has been proposed to be important for certain cancer immunotherapy antibodies.
Obesity
It has been observed that increased number of pro-inflammatory macrophages within obese adipose tissue contributes to obesity complications including insulin resistance and diabetes type 2. The modulation of the inflammatory state of adipose tissue macrophages has therefore been considered a possible therapeutic target to treat obesity-related diseases. Although adipose tissue macrophages are subject to anti-inflammatory homeostatic control by sympathetic innervation, experiments using ADRB2 gene knockout mice indicate that this effect is indirectly exerted through the modulation of adipocyte function, and not through direct Beta-2 adrenergic receptor activation, suggesting that adrenergic stimulation of macrophages may be insufficient to impact adipose tissue inflammation or function in obesity. Within the fat (adipose
Adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular ...
) tissue of CCR2
C-C chemokine receptor type 2 (CCR2 or CD192 ( cluster of differentiation 192) is a protein that in humans is encoded by the ''CCR2'' gene. CCR2 is a CC chemokine receptor.
Gene
This CCR2 gene is located in the chemokine receptor gene clust ...
deficient mice
A mouse ( : mice) is a small rodent. Characteristically, mice are known to have a pointed snout, small rounded ears, a body-length scaly tail, and a high breeding rate. The best known mouse species is the common house mouse (''Mus musculus' ...
, there is an increased number of eosinophil
Eosinophils, sometimes called eosinophiles or, less commonly, acidophils, are a variety of white blood cells (WBCs) and one of the immune system components responsible for combating multicellular parasites and certain infections in vertebrates. A ...
s, greater alternative macrophage activation, and a propensity towards type 2 cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
expression. Furthermore, this effect was exaggerated when the mice became obese
Obesity is a medical condition, sometimes considered a disease, in which excess body fat has accumulated to such an extent that it may negatively affect health. People are classified as obese when their body mass index (BMI)—a person's we ...
from a high fat diet. This is partially caused by a phenotype switch of macrophages induced by necrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated dige ...
of fat cells (adipocyte
Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. I ...
s). In an obese individual some adipocytes burst and undergo necrotic death, which causes the residential M2 macrophages to switch to M1 phenotype. This is one of the causes of a low-grade systemic chronic inflammatory state associated with obesity.
Intestinal macrophages
Though very similar in structure to tissue macrophages, intestinal macrophages have evolved specific characteristics and functions given their natural environment, which is in the digestive tract. Macrophages and intestinal macrophages have high plasticity causing their phenotype to be altered by their environments. Like macrophages, intestinal macrophages are differentiated monocytes, though intestinal macrophages have to coexist with themicrobiome
A microbiome () is the community of microorganisms that can usually be found living together in any given habitat. It was defined more precisely in 1988 by Whipps ''et al.'' as "a characteristic microbial community occupying a reasonably wel ...
in the intestines. This is a challenge considering the bacteria found in the gut are not recognized as "self" and could be potential targets for phagocytosis by the macrophage.
To prevent the destruction of the gut bacteria, intestinal macrophages have developed key differences compared to other macrophages. Primarily, intestinal macrophages do not induce inflammatory responses. Whereas tissue macrophages release various inflammatory cytokines, such as IL-1, IL-6 and TNF-α, intestinal macrophages do not produce or secrete inflammatory cytokines. This change is directly caused by the intestinal macrophages environment. Surrounding intestinal epithelial cells release TGF-β
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other sign ...
, which induces the change from proinflammatory macrophage to noninflammatory macrophage.
Even though the inflammatory response is downregulated in intestinal macrophages, phagocytosis is still carried out. There is no drop off in phagocytosis efficiency as intestinal macrophages are able to effectively phagocytize the bacteria,''S. typhimurium'' and '' E. coli'', but intestinal macrophages still do not release cytokines, even after phagocytosis. Also, intestinal macrophages do not express lipopolysaccharide (LPS), IgA, or IgG receptors. The lack of LPS receptors is important for the gut as the intestinal macrophages do not detect the microbe-associated molecular patterns (MAMPS/PAMPS) of the intestinal microbiome. Nor do they express IL-2 and IL-3 growth factor receptors.
Role in disease
Intestinal macrophages have been shown to play a role in inflammatory bowel disease (IBD), such as Crohn's disease (CD) andulcerative colitis
Ulcerative colitis (UC) is a long-term condition that results in inflammation and ulcers of the colon and rectum. The primary symptoms of active disease are abdominal pain and diarrhea mixed with blood (hematochezia). Weight loss, fever, and ...
(UC). In a healthy gut, intestinal macrophages limit the inflammatory response in the gut, but in a disease-state, intestinal macrophage numbers and diversity are altered. This leads to inflammation of the gut and disease symptoms of IBD. Intestinal macrophages are critical in maintaining gut homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
. The presence of inflammation or pathogen alters this homeostasis, and concurrently alters the intestinal macrophages. There has yet to be a determined mechanism for the alteration of the intestinal macrophages by recruitment of new monocytes or changes in the already present intestinal macrophages.
Media
interferon-γ
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheelock ...
one night before filming with conidia. Observations were made every 30s over a 2.5hr period.
File:S3-Alveolar Macrophages with Conidia in Liquid Medium.ogv, Two highly active alveolar macrophage
An alveolar macrophage, pulmonary macrophage, (or dust cell) is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.
Activity of the alveolar macropha ...
s can be seen ingesting conidia. Time lapse is 30s per frame over 2.5hr.
History
Macrophages were first discovered late in the 19th century by Élie Metchnikoff.See also
*Bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
* Dendritic cell
* Histiocyte
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system (also known as the reticuloendothelial system or lymphoreticular system). The mononuclear phagocytic system is part of the organism's immune system. The histiocyt ...
References
External links
The role of macrophages in HIV pathogenesis
Macrophages News
Macrophages News provided by insciences organisation
www.macrophages.com
The Macrophage Community Website {{Authority control Phagocytes Cell biology Immune system Human cells Articles containing video clips Connective tissue cells Lymphatic system