|
Interleukin 4
The interleukin 4 (IL4, IL-4) is a cytokine that induces differentiation of naive helper T cells ( Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils. It is closely related and has functions similar to IL-13. Function Interleukin 4 has many biological roles, including the stimulation of activated B cell and T cell proliferation, and the differentiation of B cells into plasma cells. It is a key regulator in humoral and adaptive immunity. IL-4 induces B cell class switching to IgE, and up-regulates MHC class II production. IL-4 decreases the production of Th1 cells, macrophages, IFNγ, and dendritic cells IL-12. Overproduction of IL-4 is associated with allergies. * Inflammation and wound repair Tissue macrophages play an important role in chronic inflammation and wound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors, but generally not hormones or growth factors (despite some overlap in the terminology). Cytokines are produced by a broad range of cells, including immune cells like macrophages, B lymphocytes, T lymphocytes and mast cells, as well as endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. They act through cell surface receptors and are especially important in the immune system; cytokines modulate the balance between humoral and cell-based immune responses, and they regulate the maturati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrophage
Macrophages (abbreviated as M φ, MΦ or MP) ( el, large eaters, from Greek ''μακρός'' (') = large, ''φαγεῖν'' (') = to eat) are a type of white blood cell of the immune system that engulfs and digests pathogens, such as cancer cells, microbe A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...s, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. The process is called phagocytosis, which acts to defend the host against infection and injury. These large phagocytes are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand- helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulphide Bonds
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokines
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents. Cytokines include chemokines, interferons, interleukins, lymphokines, and tumour necrosis factors, but generally not hormones or growth factors (despite some overlap in the terminology). Cytokines are produced by a broad range of cells, including immune cells like macrophages, B lymphocytes, T lymphocytes and mast cells, as well as endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. They act through cell surface receptors and are especially important in the immune system; cytokines modulate the balance between humoral and cell-based immune responses, and they regulate the maturati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a polypeptide folds into its characteristic three-dimensional structure from a random coil. Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of mRNA to a linear chain of amino acids. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a ribosome, the linear chain begins to fold into its three-dimensional structure. Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IL-13Rα1
Interleukin 13 receptor, alpha 1, also known as IL13RA1 and CD213A1 (cluster of differentiation 213A1), is a human gene. The protein encoded by this gene is a subunit of the interleukin 13 receptor. This subunit forms a receptor complex with IL4 receptor alpha, a subunit shared by IL13 and IL4 receptors. This subunit serves as a primary IL13-binding subunit of the IL13 receptor, and may also be a component of IL4 receptors. This protein has been shown to bind tyrosine kinase TYK2, and thus may mediate the signaling processes that lead to the activation of JAK1, STAT3 and STAT6 induced by IL13 and IL4. See also * Interleukin-13 receptor The interleukin-13 receptor is a type I cytokine receptor, binding Interleukin-13. It consists of two subunits, encoded by IL13RA1 and IL4R, respectively. These two genes encode the proteins IL-13Rα1 and IL-4Rα. These form a dimer with IL-13 b ... References Further reading * * * * * * * * * * * * * * * * * * * * External links * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin-4 Receptor
The interleukin 4 receptor is a type I cytokine receptor. IL4R is its human gene. Function This gene encodes the alpha chain of the interleukin-4 receptor, a type I transmembrane protein that can bind interleukin 4 and interleukin 13 to regulate IgE antibody production in B cells. Among T cells, the encoded protein also can bind interleukin 4 to promote differentiation of Th2 cells. A soluble form of the encoded protein can be produced by an alternate splice variant or by proteolysis of the membrane-bound protein, and this soluble form can inhibit IL4-mediated cell proliferation and IL5 upregulation by T-cells. Allelic variations in this gene have been associated with atopy, a condition that can manifest itself as allergic rhinitis, sinusitis, asthma, or eczema. Two transcript variants encoding different isoforms, a membrane-bound and a soluble form, have been found for this gene. Interactions of IL-4 with TNFα promote structural changes to vascular endothelial cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
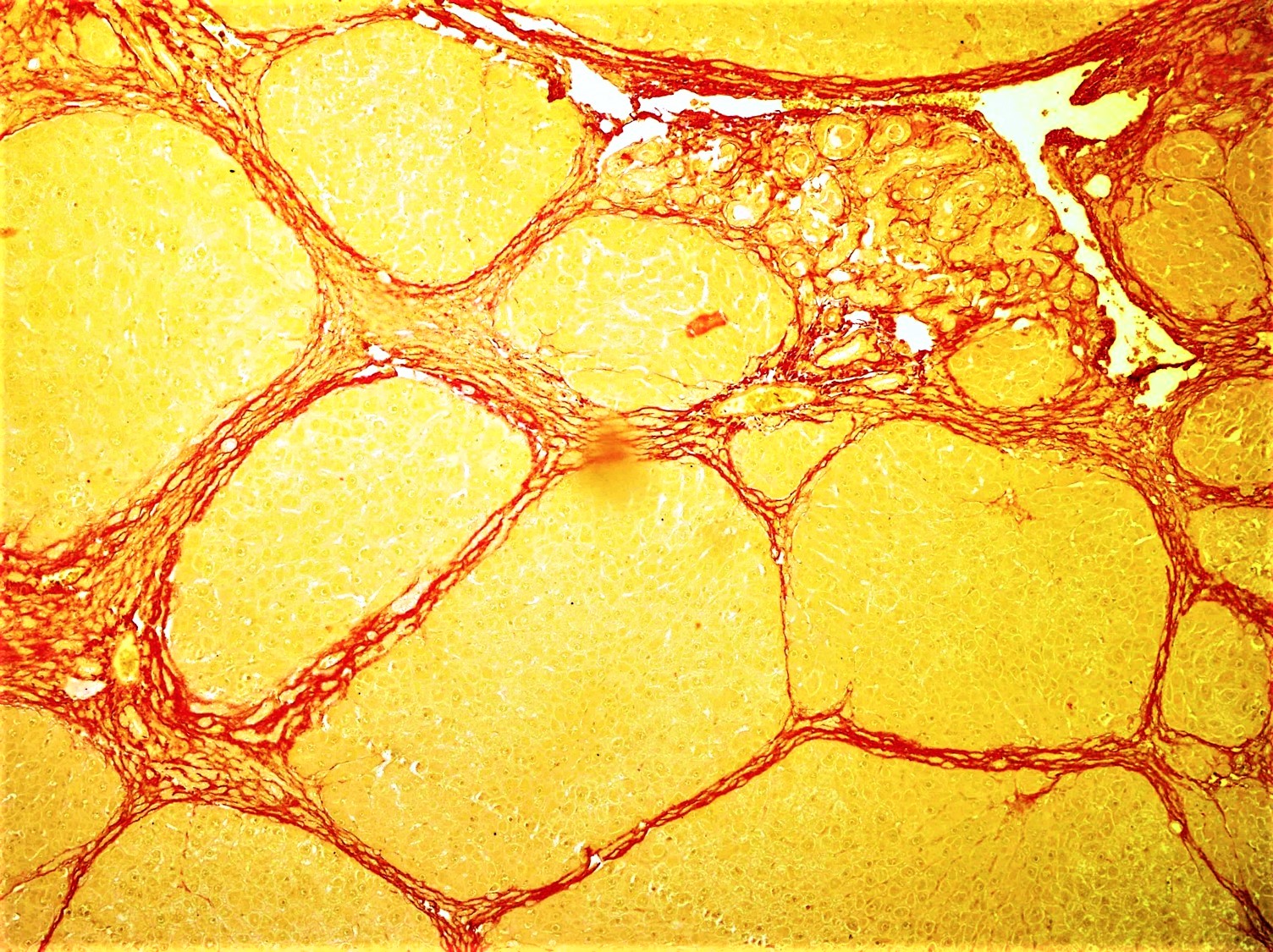
Fibrosis
Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue. Repeated injuries, chronic inflammation and repair are susceptible to fibrosis where an accidental excessive accumulation of extracellular matrix components, such as the collagen is produced by fibroblasts, leading to the formation of a permanent fibrotic scar. In response to injury, this is called scarring, and if fibrosis arises from a single cell line, this is called a fibroma. Physiologically, fibrosis acts to deposit connective tissue, which can interfere with or totally inhibit the normal architecture and function of the underlying organ or tissue. Fibrosis can be used to describe the pathological state of excess deposition of fibrous tissue, as well as the process of connective tissue deposition in healing. Define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginase
Arginase (, ''arginine amidinase'', ''canavanase'', ''L-arginase'', ''arginine transamidinase'') is a manganese-containing enzyme. The reaction catalyzed by this enzyme is: : arginine + H2O → ornithine + urea It is the final enzyme of the urea cycle. It is ubiquitous to all domains of life. Structure and function Arginase belong to the ureohydrolase family of enzymes. Arginase catalyzes the fifth and final step in the urea cycle, a series of biochemical reactions in mammals during which the body disposes of harmful ammonia. Specifically, arginase converts L-arginine into L-ornithine and urea. Mammalian arginase is active as a trimer, but some bacterial arginases are hexameric. The enzyme requires a two-molecule metal cluster of manganese in order to maintain proper function. These Mn2+ ions coordinate with water, orienting and stabilizing the molecule and allowing water to act as a nucleophile and attack L-arginine, hydrolyzing it into ornithine and urea. In most mammals, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transforming Growth Factor Beta
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other signaling proteins. TGFB proteins are produced by all white blood cell lineages. Activated TGF-β complexes with other factors to form a serine/threonine kinase complex that binds to TGF-β receptors. TGF-β receptors are composed of both type 1 and type 2 receptor subunits. After the binding of TGF-β, the type 2 receptor kinase phosphorylates and activates the type 1 receptor kinase that activates a signaling cascade. This leads to the activation of different downstream substrates and regulatory proteins, inducing transcription of different target genes that function in differentiation, chemotaxis, proliferation, and activation of many immune cells. TGF-β is secreted by many cell types, including macrophages, in a latent form in whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |