pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
loop, classifying it as a aliphatic amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring.
History and etymology
Proline was first isolated in 1900 by Richard Willstätter who obtained the amino acid while studying ''N''-methylproline, and synthesized proline by the reaction of sodium salt of diethyl malonate with 1,3-dibromopropane. The next year, Emil Fischer isolated proline from casein and the decomposition products of γ-phthalimido-propylmalonic ester, and published the synthesis of proline from phthalimide propylmalonic ester. The name proline comes frompyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most ...
, one of its constituents.
Biosynthesis
Proline is biosynthetically derived from the amino acid L-glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
. Glutamate-5-semialdehyde is first formed by glutamate 5-kinase (ATP-dependent) and glutamate-5-semialdehyde dehydrogenase (which requires NADH or NADPH). This can then either spontaneously cyclize to form 1-pyrroline-5-carboxylic acid, which is reduced to proline by pyrroline-5-carboxylate reductase (using NADH or NADPH), or turned into ornithine by ornithine aminotransferase, followed by cyclisation by ornithine cyclodeaminase to form proline.

Biological activity
L-Proline has been found to act as a weak agonist of theglycine receptor
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory ...
and of both NMDA and non-NMDA ( AMPA/ kainate) ionotropic glutamate receptors. It has been proposed to be a potential endogenous
Endogenous substances and processes are those that originate from within a living system such as an organism, tissue, or cell.
In contrast, exogenous substances and processes are those that originate from outside of an organism.
For example, es ...
excitotoxin. In plants, proline accumulation is a common physiological response to various stresses but is also part of the developmental program in generative tissues
Generative may refer to:
* Generative actor, a person who instigates social change
* Generative art, art that has been created using an autonomous system that is frequently, but not necessarily, implemented using a computer
* Generative music, mus ...
(e.g. pollen
Pollen is a powdery substance produced by seed plants. It consists of pollen grains (highly reduced microgametophytes), which produce male gametes (sperm cells). Pollen grains have a hard coat made of sporopollenin that protects the gametophyt ...
).
A diet rich in proline was linked to an increased risk of depression in humans in a study from 2022 that was tested on a limited pre-clinical trial on humans and primarily in other organisms. Results were significant in the other organisms.
Properties in protein structure
The distinctive cyclic structure of proline's side chain gives proline an exceptional conformational rigidity compared to other amino acids. It also affects the rate of peptide bond formation between proline and other amino acids. When proline is bound as an amide in a peptide bond, its nitrogen is not bound to any hydrogen, meaning it cannot act as ahydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
donor, but can be a hydrogen bond acceptor.
Peptide bond formation with incoming Pro-tRNAPro is considerably slower than with any other tRNAs, which is a general feature of ''N''-alkylamino acids. Peptide bond formation is also slow between an incoming tRNA and a chain ending in proline; with the creation of proline-proline bonds slowest of all.
The exceptional conformational rigidity of proline affects the secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
of proteins near a proline residue and may account for proline's higher prevalence in the proteins of thermophilic organisms. Protein secondary structure
Protein secondary structure is the three dimensional form of ''local segments'' of proteins. The two most common secondary structural elements are alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure ...
can be described in terms of the dihedral angles φ, ψ and ω of the protein backbone. The cyclic structure of proline's side chain locks the angle φ at approximately −65°.
Proline acts as a structural disruptor in the middle of regular secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
elements such as alpha helices and beta sheets; however, proline is commonly found as the first residue of an alpha helix and also in the edge strands of beta sheets. Proline is also commonly found in turns (another kind of secondary structure), and aids in the formation of beta turns. This may account for the curious fact that proline is usually solvent-exposed, despite having a completely aliphatic side chain.
Multiple prolines and/or hydroxyprolines in a row can create a polyproline helix, the predominant secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
in collagen. The hydroxylation
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a ...
of proline by prolyl hydroxylase (or other additions of electron-withdrawing substituents such as fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
) increases the conformational stability of collagen significantly. Hence, the hydroxylation of proline is a critical biochemical process for maintaining the connective tissue of higher organisms. Severe diseases such as scurvy can result from defects in this hydroxylation, e.g., mutations in the enzyme prolyl hydroxylase or lack of the necessary ascorbate (vitamin C) cofactor.
''Cis''–''trans'' isomerization
Peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
s to proline, and to other ''N''-substituted amino acids (such as sarcosine), are able to populate both the '' cis'' and '' trans'' isomers. Most peptide bonds overwhelmingly adopt the ''trans'' isomer (typically 99.9% under unstrained conditions), chiefly because the amide hydrogen (''trans'' isomer) offers less steric repulsion to the preceding Cα atom than does the following Cα atom (''cis'' isomer). By contrast, the ''cis'' and ''trans'' isomers of the X-Pro peptide bond (where X represents any amino acid) both experience steric clashes with the neighboring substitution and have a much lower energy difference. Hence, the fraction of X-Pro peptide bonds in the ''cis'' isomer under unstrained conditions is significantly elevated, with ''cis'' fractions typically in the range of 3-10%. However, these values depend on the preceding amino acid, with Gly and aromatic residues yielding increased fractions of the ''cis'' isomer. ''Cis'' fractions up to 40% have been identified for aromatic–proline peptide bonds.
From a kinetic standpoint, ''cis''–''trans'' proline isomerization is a very slow process that can impede the progress of protein folding by trapping one or more proline residues crucial for folding in the non-native isomer, especially when the native protein requires the ''cis'' isomer. This is because proline residues are exclusively synthesized in the ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to ...
as the ''trans'' isomer form. All organisms possess prolyl isomerase enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
s to catalyze this isomerization, and some bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
have specialized prolyl isomerases associated with the ribosome. However, not all prolines are essential for folding, and protein folding may proceed at a normal rate despite having non-native conformers of many X–Pro peptide bonds.
Uses
Proline and its derivatives are often used as asymmetric catalysts inproline organocatalysis Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated. Modifications, such as ...
reactions. The CBS reduction
CBS Broadcasting Inc., commonly shortened to CBS, the abbreviation of its former legal name Columbia Broadcasting System, is an American commercial broadcast television and radio network serving as the flagship property of the CBS Entertainmen ...
and proline catalysed aldol condensation are prominent examples.
In brewing, proteins rich in proline combine with polyphenols to produce haze (turbidity).
L-Proline is an osmoprotectant and therefore is used in many pharmaceutical and biotechnological applications.
The growth medium
A growth medium or culture medium is a solid, liquid, or semi-solid designed to support the growth of a population of microorganisms or cells via the process of cell proliferation or small plants like the moss ''Physcomitrella patens''. Differen ...
used in plant tissue culture may be supplemented with proline. This can increase growth, perhaps because it helps the plant tolerate the stresses of tissue culture. For proline's role in the stress response of plants, see .
Specialties
Proline is one of the two amino acids that do not follow along with the typical Ramachandran plot, along with glycine. Due to the ring formation connected to the beta carbon, the ''ψ'' and ''φ'' angles about the peptide bond have fewer allowable degrees of rotation. As a result, it is often found in "turns" of proteins as its free entropy (Δ''S'') is not as comparatively large to other amino acids and thus in a folded form vs. unfolded form, the change in entropy is smaller. Furthermore, proline is rarely found in α and β structures as it would reduce the stability of such structures, because its side chain α-nitrogen can only form one nitrogen bond. Additionally, proline is the only amino acid that does not form a red-purple colour when developed by spraying with ninhydrin for uses in chromatography. Proline, instead, produces an orange-yellow colour.Synthesis
Racemic proline can be synthesized from diethyl malonate and acrylonitrile:Vogel, ''Practical Organic Chemistry'' 5th edition :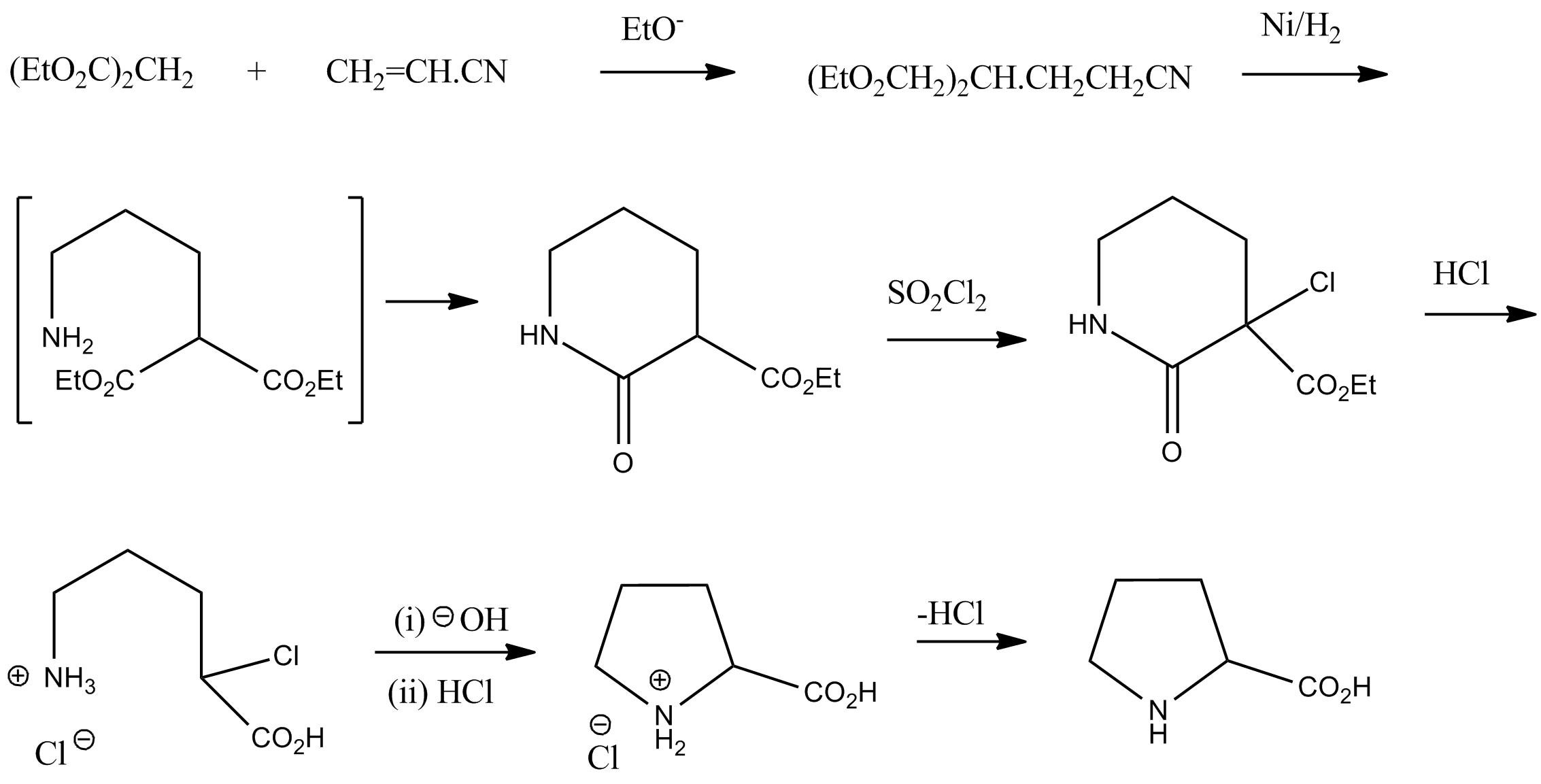
See also
*Hyperprolinemia
Hyperprolinemia is a condition which occurs when the amino acid proline is not broken down properly by the enzymes proline oxidase or 1-Pyrroline-5-carboxylate dehydrogenase, pyrroline-5-carboxylate dehydrogenase, causing a buildup of proline in t ...
* Inborn error of metabolism
* Prolidase deficiency
* Prolinol
References
Further reading
* .External links
Proline MS Spectrum
Proline biosynthesis
{{Authority control AMPA receptor agonists Kainate receptor agonists NMDA receptor agonists Proteinogenic amino acids Glucogenic amino acids Glycine receptor agonists Cyclic amino acids Pyrrolidines Secondary amino acids Excitatory amino acids