|
Ornithine Aminotransferase
Ornithine aminotransferase (OAT) is an enzyme which is encoded in human by the OAT gene located on chromosome 10. The OAT involved in the ultimate formation of the non-essential amino acid proline from the amino acid ornithine. Ornithine aminotransferase forms the initial intermediate in this process. It catalyzes the reverse reaction as well, and is therefore essential in creating ornithine from the starting substrate proline. Structure The OAT gene encodes for a protein that is approximately 46 kDa in size. The OAT protein is expressed primarily in the liver and the kidney but also in the brain and the retina. The OAT protein is localized to the mitochondrion within the cells where it is expressed. The structure of the OAT protein has been resolved using X-ray crystallography and shows similarity to other subgroup 2 aminotransferases such as dialkyglucine decarboxylatse. The OAT protein functions as a dimer and each monomer consists of a large domain, which contributes mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (chemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate-5-semialdehyde
Glutamate-5-semialdehyde is a non-proteinogenic amino acid involved in both the biosynthesis and degradation of proline and arginine (via ornithine), as well as in the biosynthesis of antibiotics, such as carbapenems. It is synthesized by the reduction of glutamyl-5-phosphate by glutamate-5-semialdehyde dehydrogenase. See also * Glutamate-1-semialdehyde Glutamate-1-semialdehyde is a molecule formed from by the reduction of tRNA bound glutamate, catalyzed by glutamyl-tRNA reductase. It is isomerized by glutamate-1-semialdehyde 2,1-aminomutase to give aminolevulinic acid in the biosynthesis of por ... References {{Amino acid metabolism intermediates Amino acids Aldehydes Aldehydic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
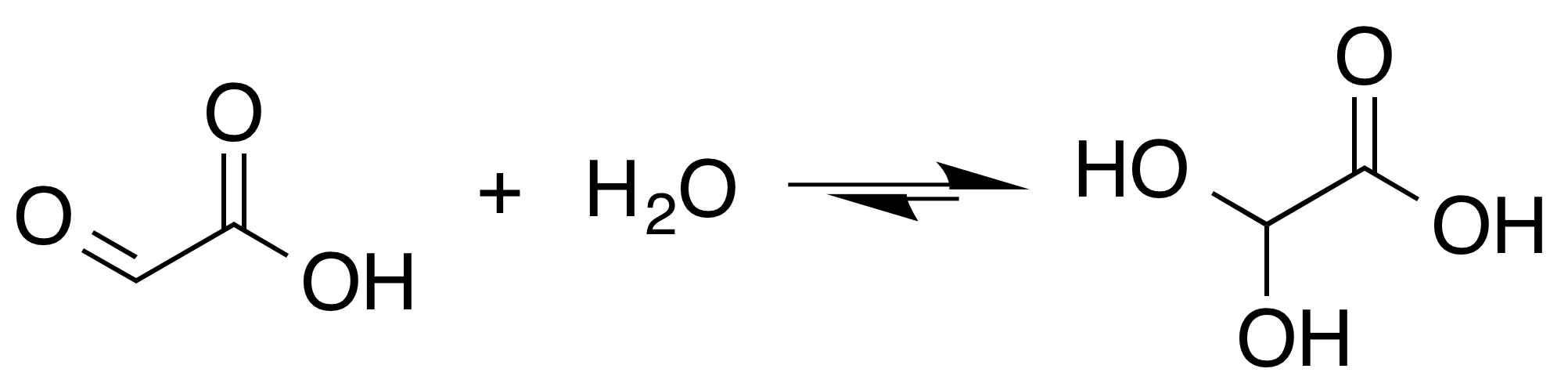
Glyoxylate
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature Although the structure of glyoxylic acid is described as having an aldehyde functional group, the aldehyde is only a minor component of the form most prevalent in some situations. Instead, it often exists as a hydrate or a cyclic dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: : In solution, the monohydrate exists in equilibrium with a hemi acylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. : In isolation, the aldehyde structure ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Aminotransferase Deficiency
Ornithine aminotransferase deficiency (also known as gyrate atrophy of the choroid and retina) is an inborn error of ornithine metabolism, caused by decreased activity of the enzyme ornithine aminotransferase. Biochemically, it can be detected by elevated levels of ornithine in the blood. Clinically, it presents initially with poor night vision, which slowly progresses to total blindness. It is believed to be inherited in an autosomal recessive manner. Approximately 200 known cases have been reported in the literature. The incidence is highest in Finland, estimated at 1:50,000. Research suggests there can be some adverse effect on muscles and also the brain. The cause of this is somewhat unclear but may relate to very low levels of creatine often found in this population. Treatment may include vitamin B6, lysine or dramatic dietary change to minimise arginine from patients diet. Research has indicated that these treatments may be somewhat effective in lowering ornithine blood ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate 5-semialdehyde
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxal 5'-phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates. Role as a coenzyme PLP acts as a coenzyme in all transamination reactions, and in certain decarboxylation, deamination, and racemization reactions of amino acids. The aldehyde group of PLP forms a Schiff-base linkage (internal aldimine) with the ε-amino group of a specific lysine group of the aminotransferase enzyme. The α-amino group of the amino acid substrate displaces the ε-amino group of the active-site lysine residue in a process known as transaldimination. The resulting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) and residues that catalyse a reaction of that substrate (catalytic site). Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes. Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their function. The active si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schiff Base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. where R' ≠ H). A number of special naming systems exist for these compounds. For instance a Schiff base derived from an aniline, where is a phenyl or a substituted phenyl, can be called an ''anil'', while bis-compounds are often referred to as salen-type compounds. The term Schiff base is normally applied to these compounds when they are being used as ligands to form coordination complexes with metal ions. Such complexes occur naturally, for instance in corrin, but the majority of Schiff bases are artificial and are used to form many important catalysts, such as Jacobsen's catalyst. Synthesis Schiff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |