Krypton (other) on:
[Wikipedia]
[Google]
[Amazon]
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the
 Krypton was discovered in Britain in 1898 by William Ramsay, a Scottish chemist, and Morris Travers, an English chemist, in residue left from evaporating nearly all components of liquid air.
Krypton was discovered in Britain in 1898 by William Ramsay, a Scottish chemist, and Morris Travers, an English chemist, in residue left from evaporating nearly all components of liquid air.
Kr + F2 -> KrF2
Krypton gas in a krypton fluoride laser absorbs energy from a source, causing the krypton to react with fluorine gas, producing the exciplex krypton fluoride, a temporary complex in an excited energy state:
:2Kr + F2 -> 2KrF
The complex can undergo spontaneous or stimulated emission, reducing its energy state to a metastable, but highly repulsive ground state. The ground state complex quickly dissociates into unbound atoms:
:2KrF -> 2Kr + F2
The result is an 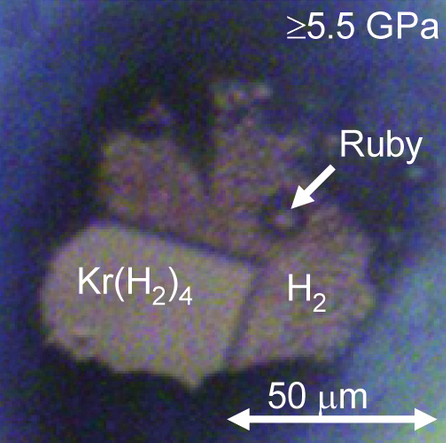
 Compounds with krypton bonded to atoms other than
Compounds with krypton bonded to atoms other than
 Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography. Krypton gas is also combined with mercury to make luminous signs that glow with a bright greenish-blue light.
Krypton is mixed with argon in energy efficient fluorescent lamps, reducing the power consumption, but also reducing the light output and raising the cost. Krypton costs about 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher
Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography. Krypton gas is also combined with mercury to make luminous signs that glow with a bright greenish-blue light.
Krypton is mixed with argon in energy efficient fluorescent lamps, reducing the power consumption, but also reducing the light output and raising the cost. Krypton costs about 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher
 Krypton is considered to be a non-toxic
Krypton is considered to be a non-toxic
"Krypton 85: a Review of the Literature and an Analysis of Radiation Hazards"
Environmental Protection Agency, Office of Research and Monitoring, Washington (1972)
at '' The Periodic Table of Videos'' (University of Nottingham)
Krypton Fluoride Lasers
Plasma Physics Division Naval Research Laboratory {{Krypton compounds Chemical elements Noble gases
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts
''Trace Amounts: Autism, Mercury, and the Hidden Truth'' is a 2014 anti-vaccination biographic film by Eric Gladen, who claims to have experienced mercury poisoning after receiving a tetanus vaccine. In the film, he presents his investigation on ...
in the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert
In chemistry, the term chemically inert is used to describe a substance that is not chemically reactive. From a thermodynamic perspective, a substance is inert, or nonlabile, if it is thermodynamically unstable (positive standard Gibbs free en ...
.
Krypton, like the other noble gases, is used in lighting and photography. Krypton light has many spectral lines, and krypton plasma
Plasma or plasm may refer to:
Science
* Plasma (physics), one of the four fundamental states of matter
* Plasma (mineral), a green translucent silica mineral
* Quark–gluon plasma, a state of matter in quantum chromodynamics
Biology
* Blood pla ...
is useful in bright, high-powered gas lasers (krypton ion and excimer lasers), each of which resonates and amplifies a single spectral line. Krypton fluoride also makes a useful laser medium. From 1960 to 1983, the official definition of meter was based on the wavelength of one spectral line of krypton-86, because of the high power and relative ease of operation of krypton discharge tubes.
History
 Krypton was discovered in Britain in 1898 by William Ramsay, a Scottish chemist, and Morris Travers, an English chemist, in residue left from evaporating nearly all components of liquid air.
Krypton was discovered in Britain in 1898 by William Ramsay, a Scottish chemist, and Morris Travers, an English chemist, in residue left from evaporating nearly all components of liquid air. Neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
was discovered by a similar procedure by the same workers just a few weeks later. William Ramsay was awarded the 1904 Nobel Prize in Chemistry for discovery of a series of noble gases, including krypton.
In 1960, the International Bureau of Weights and Measures defined the meter as 1,650,763.73 wavelengths of light emitted in the vacuum corresponding to the transition between the 2p10 and 5d5 levels in the isotope krypton-86. This agreement replaced the 1889 international prototype meter, which was a metal bar located in Sèvres. This also obsoleted the 1927 definition of the ångström based on the red cadmium spectral line, replacing it with 1 Å = 10−10 m. The krypton-86 definition lasted until the October 1983 conference, which redefined the meter as the distance that light travels in vacuum during 1/299,792,458 s.
Characteristics
Krypton is characterized by several sharp emission lines ( spectral signatures) the strongest being green and yellow. Krypton is one of the products of uraniumfission
Fission, a splitting of something into two or more parts, may refer to:
* Fission (biology), the division of a single entity into two or more parts and the regeneration of those parts into separate entities resembling the original
* Nuclear fissio ...
. Solid krypton is white and has a face-centered cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
crystal structure, which is a common property of all noble gases (except helium, which has a hexagonal close-packed crystal structure).
Isotopes
Naturally occurring krypton in Earth's atmosphere is composed of fivestable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
isotopes, plus one isotope (78Kr) with such a long half-life (9.2×1021 years) that it can be considered stable. (This isotope has the second-longest known half-life among all isotopes for which decay has been observed; it undergoes double electron capture to 78 Se). In addition, about thirty unstable isotopes and isomers are known. Traces of 81Kr, a cosmogenic nuclide produced by the cosmic ray irradiation of 80Kr, also occur in nature: this isotope is radioactive with a half-life of 230,000 years. Krypton is highly volatile and does not stay in solution in near-surface water, but 81Kr has been used for dating old (50,000–800,000 years) groundwater.
85Kr is an inert radioactive noble gas with a half-life of 10.76 years. It is produced by the fission
Fission, a splitting of something into two or more parts, may refer to:
* Fission (biology), the division of a single entity into two or more parts and the regeneration of those parts into separate entities resembling the original
* Nuclear fissio ...
of uranium and plutonium, such as in nuclear bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions (thermonuclear bomb), producing a nuclear explosion. Both bomb ...
testing and nuclear reactors. 85Kr is released during the reprocessing of fuel rods from nuclear reactors. Concentrations at the North Pole are 30% higher than at the South Pole due to convective mixing.
Chemistry
Like the other noble gases, krypton is chemically highly unreactive. The rather restricted chemistry of krypton in the +2 oxidation state parallels that of the neighboring element bromine in the +1 oxidation state; due to thescandide contraction
The d-block contraction (sometimes called scandide contraction) is a term used in chemistry to describe the effect of having full d orbitals on the period 4 elements. The elements in question are gallium, germanium, arsenic, selenium, bromine, ...
it is difficult to oxidize the 4p elements to their group oxidation states. Until the 1960s no noble gas compounds had been synthesized.
Following the first successful synthesis of xenon compounds in 1962, synthesis of krypton difluoride () was reported in 1963. In the same year, was reported by Grosse, ''et al.'', but was subsequently shown to be a mistaken identification. Under extreme conditions, krypton reacts with fluorine to form KrF2 according to the following equation:
:exciplex laser
An excimer laser, sometimes more correctly called an exciplex laser, is a form of ultraviolet laser which is commonly used in the production of microelectronic devices, semiconductor based integrated circuits or "chips", eye surgery, and microma ...
which radiates energy at 248 nm, near the ultraviolet portion of the spectrum, corresponding with the energy difference between the ground state and the excited state of the complex.

 Compounds with krypton bonded to atoms other than
Compounds with krypton bonded to atoms other than fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
have also been discovered. There are also unverified reports of a barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
salt of a krypton oxoacid. ArKr+ and Kr H+ polyatomic ions
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not ...
have been investigated and there is evidence for Kr Xe or KrXe+.
The reaction of with produces an unstable compound, , that contains a krypton- oxygen bond. A krypton- nitrogen bond is found in the cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
C≡N–Kr–F produced by the reaction of with C≡NHAsF] below −50 °C. HKrCN and HKrC≡CH (krypton hydride-cyanide and hydrokryptoacetylene) were reported to be stable up to 40 kelvin, K.
Krypton hydride (Kr(H2)4) crystals can be grown at pressures above 5 GPa. They have a face-centered cubic structure where krypton octahedra are surrounded by randomly oriented hydrogen molecules.
Natural occurrence
Earth has retained all of the noble gases that were present at its formation except helium. Krypton's concentration in theatmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
is about 1 ppm. It can be extracted from liquid air by fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to ...
. The amount of krypton in space is uncertain, because measurement is derived from meteoric activity and solar winds. The first measurements suggest an abundance of krypton in space.
Applications
 Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography. Krypton gas is also combined with mercury to make luminous signs that glow with a bright greenish-blue light.
Krypton is mixed with argon in energy efficient fluorescent lamps, reducing the power consumption, but also reducing the light output and raising the cost. Krypton costs about 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher
Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a white light source. Krypton is used in some photographic flashes for high speed photography. Krypton gas is also combined with mercury to make luminous signs that glow with a bright greenish-blue light.
Krypton is mixed with argon in energy efficient fluorescent lamps, reducing the power consumption, but also reducing the light output and raising the cost. Krypton costs about 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher operating temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the de ...
s.
Krypton's white discharge is sometimes used as an artistic effect in gas discharge "neon" tubes. Krypton produces much higher light power than neon in the red spectral line region, and for this reason, red lasers for high-power laser light-shows are often krypton lasers with mirrors that select the red spectral line for laser amplification and emission, rather than the more familiar helium-neon variety, which could not achieve the same multi-watt outputs.
The krypton fluoride laser is important in nuclear fusion energy research in confinement experiments. The laser has high beam uniformity, short wavelength, and the spot size can be varied to track an imploding pellet.
In experimental particle physics, liquid krypton is used to construct quasi-homogeneous electromagnetic calorimeters. A notable example is the calorimeter of the NA48
The NA48 experiment was a series of particle physics experiments in the field of kaon physics being carried out at the North Area of the Super Proton Synchrotron at CERN. The collaboration involved over 100 physicists mostly from Western Europe ...
experiment at CERN
The European Organization for Nuclear Research, known as CERN (; ; ), is an intergovernmental organization that operates the largest particle physics laboratory in the world. Established in 1954, it is based in a northwestern suburb of Gene ...
containing about 27 tonnes of liquid krypton. This usage is rare, since liquid argon is less expensive. The advantage of krypton is a smaller Molière radius
The Molière radius is a characteristic constant of a material giving the scale of the transverse dimension of the fully contained electromagnetic showers initiated by an incident high energy electron or photon. By definition, it is the radius of ...
of 4.7 cm, which provides excellent spatial resolution with little overlapping. The other parameters relevant for calorimetry are: radiation length of X0=4.7 cm, and density of 2.4 g/cm3.
Krypton-83 has application in magnetic resonance imaging
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio wave ...
(MRI) for imaging airways. In particular, it enables the radiologist to distinguish between hydrophobic and hydrophilic surfaces containing an airway.
Although xenon has potential for use in computed tomography
A computed tomography scan (CT scan; formerly called computed axial tomography scan or CAT scan) is a medical imaging technique used to obtain detailed internal images of the body. The personnel that perform CT scans are called radiographers ...
(CT) to assess regional ventilation, its anesthetic properties limit its fraction in the breathing gas to 35%. A breathing mixture of 30% xenon and 30% krypton is comparable in effectiveness for CT to a 40% xenon fraction, while avoiding the unwanted effects of a high partial pressure of xenon gas. The metastable isotope krypton-81m is used in nuclear medicine for lung ventilation/perfusion scans, where it is inhaled and imaged with a gamma camera. Krypton-85 in the atmosphere has been used to detect clandestine nuclear fuel reprocessing facilities in North Korea and Pakistan. Those facilities were detected in the early 2000s and were believed to be producing weapons-grade plutonium. Krypton-85 is a medium lived fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
and thus escapes from spent fuel when the cladding is removed.
Krypton is used occasionally as an insulating gas between window panes. SpaceX
Space Exploration Technologies Corp. (SpaceX) is an American spacecraft manufacturer, launcher, and a satellite communications corporation headquartered in Hawthorne, California. It was founded in 2002 by Elon Musk with the stated goal of ...
Starlink use krypton as propellant for their electric propulsion system.
Precautions
 Krypton is considered to be a non-toxic
Krypton is considered to be a non-toxic asphyxiant
An asphyxiant gas, also known as a simple asphyxiant, is a nontoxic or minimally toxic gas which reduces or displaces the normal oxygen concentration in breathing air. Breathing of oxygen-depleted air can lead to death by asphyxiation (suffocation) ...
. Being lipophilic, krypton has a significant anaesthetic effect (although the mechanism of this phenomenon is still not fully clear, there is good evidence that the two properties are mechanistically related), with narcotic potency seven times greater than air, and breathing an atmosphere of 50% krypton and 50% natural air (as might happen in the locality of a leak) causes narcosis in humans similar to breathing air at four times atmospheric pressure. This is comparable to scuba diving at a depth of and could affect anyone breathing it.
References
Further reading
* William P. Kir"Krypton 85: a Review of the Literature and an Analysis of Radiation Hazards"
Environmental Protection Agency, Office of Research and Monitoring, Washington (1972)
External links
at '' The Periodic Table of Videos'' (University of Nottingham)
Krypton Fluoride Lasers
Plasma Physics Division Naval Research Laboratory {{Krypton compounds Chemical elements Noble gases