|
Chemically Inert
In chemistry, the term chemically inert is used to describe a substance that is not chemically reactive. From a thermodynamic perspective, a substance is inert, or nonlabile, if it is thermodynamically unstable (positive standard Gibbs free energy of formation) yet decomposes at a slow, or negligible rate. Most of the noble gases, which appear in the last column of the periodic table, are classified as inert (or unreactive). These elements are stable in their naturally occurring form (gaseous form) and they are called inert gases. Noble gas The noble gases ( helium, neon, argon, krypton, xenon and radon) were previously known as 'inert gases' because of their perceived lack of participation in any chemical reactions. The reason for this is that their outermost electron shells (valence shells) are completely filled, so that they have little tendency to gain or lose electrons. They are said to acquire a noble gas configuration, or a full electron configuration. It is no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work (pharmacology), and how to collect DNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
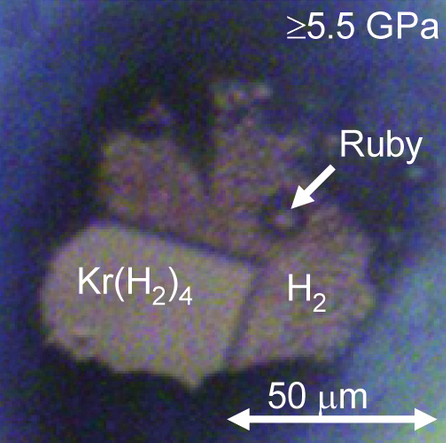
Noble Gas Compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton (ionisation energy 14.0 eV), xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of 40 K or lower, in supersonic jets of noble gas, or under extremely high pressures with metals. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water-reactive Substances
Water-reactive substances are those that spontaneously undergo a chemical reaction with water, as they are highly reducing in nature. Notable examples include alkali metals, lithium through caesium, and alkaline earth metals, magnesium through barium. Some water-reactive substances are also pyrophoric, like organometallics and sulphuric acid, and should be kept away from moisture. The use of acid-resistant gloves and face shield are required and should be handled in fume hoods. Such substances are classified as R2 under the UN classification system and as Hazard 4.3 by the United States Department of Transportation. In an NFPA 704 fire diamond's white square, they are denoted as "W̶". All chemicals that react vigorously with water or liberate toxic gas when in contact with water are recognized for their hazardous nature in the 'Approved Supply List,' or the list of substances covered by the international legislation on major hazards many of which are commonly used in manufactu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air Sensitive
Air sensitivity is a term used, particularly in chemistry, to denote the reactivity of chemical compounds with some constituent of air. Most often, reactions occur with atmospheric oxygen (O2) or water vapor (H2O), although reactions with the other constituents of air such as carbon monoxide (CO), carbon dioxide (CO2), and nitrogen (N2) are also possible. Method A variety of air-free techniques have been developed to handle air-sensitive compounds. Two main types of equipment are gloveboxes and Schlenk lines. Glove boxes are sealed cabinets filled with an inert gas such as argon or nitrogen. Normal laboratory equipment can be set up in the glovebox, and manipulated by the use of gloves that penetrate its walls. The atmosphere can be regulated to approximately atmospheric pressure and set to be pure nitrogen or other gas with which the chemicals will not react. Chemicals and equipment can be transferred in and out via an airlock. A Schlenk line is a vacuum and inert-gas dual-ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haber Process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures: : \ce \quad \Delta H^\circ = -91.8~\text Though this reaction is exothermic (i.e. it releases energy, albeit not very much), it results in a decrease in entropy, which is the central reason why it is very challenging to carry out. Before the development of the Haber process, it had been difficult to produce ammonia on an industrial scale, with early methods, such as the Birkeland–Eyde process and the Frank–Caro process, all highly inefficient. During World War I, the Haber process provided Germany with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Nitride
Lithium nitride is a compound with the formula Li3N. It is the only stable alkali metal nitride. The solid has a reddish-pink color and high melting point. Preparation and handling Lithium nitride is prepared by direct combination of elemental lithium with nitrogen gas: :6 Li + N2 → 2 Li3N Instead of burning lithium metal in an atmosphere of nitrogen, a solution of lithium in liquid sodium metal can be treated with N2. Lithium nitride reacts violently with water to produce ammonia: :Li3N + 3 H2O → 3 LiOH + NH3 Structure and properties ''alpha''-Li3N (stable at room temperature and pressure) has an unusual crystal structure that consists of two types of layers, one sheet has the composition Li2N− contains 6-coordinate N centers and the other sheet consists only of lithium cations. Two other forms are known: ''beta''-Lithium nitride, formed from the alpha phase at has the sodium arsenide (Na3As) structure; ''gamma''-Lithium nitride (same structure as Li3Bi) forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share "valence" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatomic Molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Otherwise, if a diatomic molecule consists of two different atoms, such as carbon monoxide () or nitric oxide (), the molecule is said to be heteronuclear. The bond in a homonuclear diatomic molecule is non-polar. The only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (STP) (or typical laboratory conditions of 1 bar and 25 °C) are the gases hydrogen (), nitrogen (), oxygen (), fluorine (), and chlorine (). The noble gases (helium, neon, argon, krypton, xenon, and radon) are also gases at STP, but they are monatomic. The homonuclear diatomic gases and noble gases together are called "elemental gases" or "molecular gases", to distinguish them from other gases that are chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inert Gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to as the inert gases. Inert gases are used generally to avoid unwanted chemical reactions degrading a sample. These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and moisture in air. The term ''inert gas'' is context-dependent because several of the noble gases can be made to react under certain conditions. Purified argon gas is the most commonly used inert gas due to its high natural abundance (78.3% N2, 1% Ar in air) and low relative cost. Unlike noble gases, an inert gas is not necessarily elemental and is often a compound gas. Like the noble gases, the tendency for non-reactivity is due to the valence, the outermost electron shell, being complete in all the inert gases. This is a tendency, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)


